AI-Driven DBTL Cycle: Accelerating Precision Biological Engineering and Drug Discovery
This article explores the transformative role of the Design-Build-Test-Learn (DBTL) cycle in modern biological engineering.
AI-Driven DBTL Cycle: Accelerating Precision Biological Engineering and Drug Discovery
Abstract
This article explores the transformative role of the Design-Build-Test-Learn (DBTL) cycle in modern biological engineering. Tailored for researchers and drug development professionals, it details how this iterative framework, supercharged by artificial intelligence and automation, is overcoming traditional R&D bottlenecks. We cover the foundational principles of DBTL, its methodological applications in strain engineering and cell therapy development, advanced strategies for optimizing its efficiency, and a comparative analysis of its validation in both industrial biomanufacturing and clinical research. The synthesis provides a roadmap for leveraging DBTL to achieve high-precision biological design and accelerate the development of next-generation therapeutics.
The DBTL Cycle: The Foundational Engine of Modern Synthetic Biology
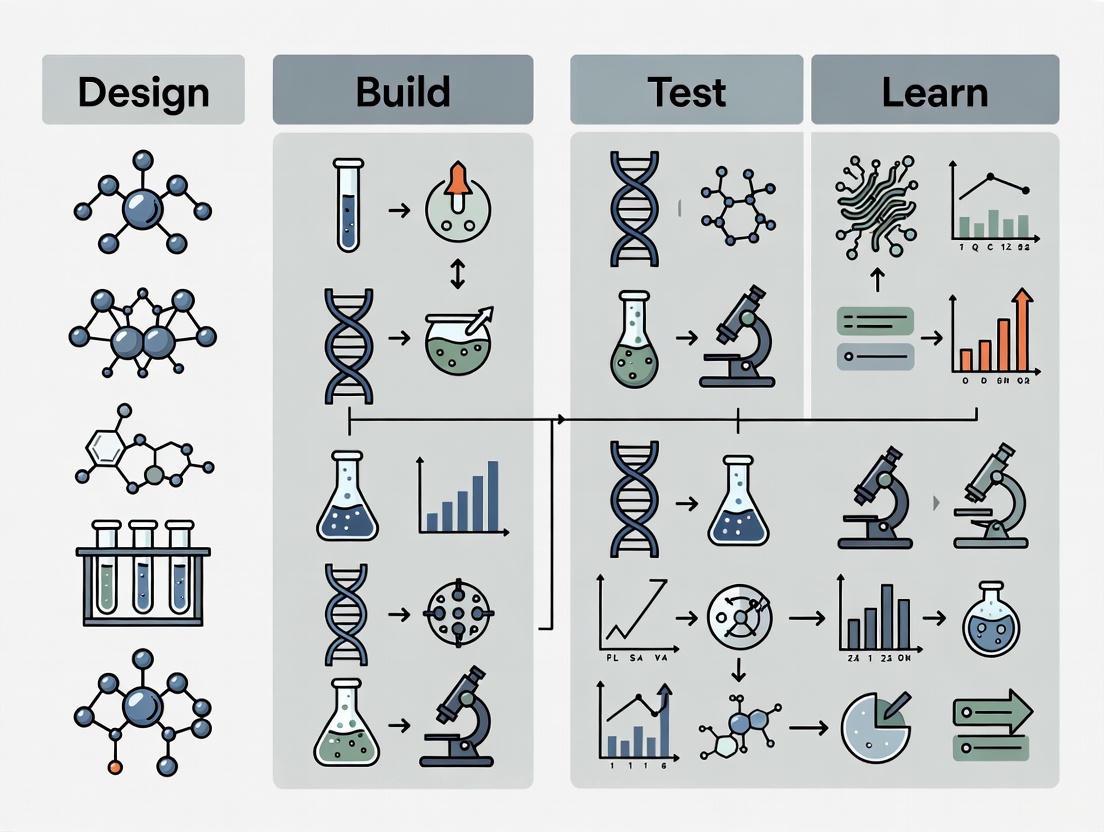
The Design-Build-Test-Learn (DBTL) cycle is a systematic, iterative framework that serves as the cornerstone of modern synthetic biology and biological engineering. This engineering mantra provides a structured methodology for developing and optimizing biological systems, enabling researchers to engineer organisms for specific functions such as producing biofuels, pharmaceuticals, or other valuable compounds [1]. The cycle begins with researchers defining objectives for desired biological function and designing相应的 biological parts or systems, which can include introducing novel components or redesigning existing parts for new applications [2]. This foundational approach mirrors established engineering disciplines, where iteration involves gathering information, processing it, identifying design revisions, and implementing those changes [2].
The power of the DBTL framework lies in its recursive nature, which streamlines and simplifies efforts to build biological systems. Through repeated cycles, researchers can progressively refine their biological constructs until they achieve the desired performance or function [1]. This iterative process has become increasingly important as synthetic biology ambitions have grown more complex, evolving from simple genetic modifications to extensive pathway engineering and whole-genome editing. The framework's flexibility allows it to be applied across various biological systems, from bacterial chassis to eukaryotic cells, mammalian systems, and cell-free platforms [2].
The Core Components of the Traditional DBTL Cycle
Design Phase
The Design phase constitutes the foundational planning stage where researchers define specific objectives and create blueprints for biological systems. This phase relies heavily on domain knowledge, expertise, and computational modeling approaches [2]. During design, researchers select and arrange biological parts—such as promoters, coding sequences, and terminators—using principles of modularity and standardization that enable the assembly of diverse genetic constructs through interchangeable components [1]. The design process must account for numerous factors, including promoter strengths, ribosome binding site sequences, codon usage biases, and secondary structure propensities, all of which influence the eventual functionality of the engineered biological system [3]. Computational tools and bioinformatics resources play an increasingly vital role in this phase, allowing researchers to model and simulate system behavior before moving to physical implementation.
Build Phase
In the Build phase, digital designs transition into physical biological entities. This stage involves the synthesis of DNA constructs, their assembly into plasmids or other vectors, and introduction into characterization systems [2]. Traditional building methods include various molecular cloning techniques, such as restriction enzyme-based assembly, Gibson assembly, and Golden Gate assembly, each offering different advantages in terms of efficiency, fidelity, and scalability [1]. The Build phase has been significantly accelerated through automation, with robotic systems enabling the assembly of a greater variety of potential constructs by interchanging individual components [1]. More recently, innovative approaches like sequencing-free cloning that leverage Golden Gate Assembly with vectors containing "suicide genes" have achieved cloning accuracy of nearly 90%, eliminating the need for time-consuming colony picking and sequence verification [4]. Build outputs are typically verified using colony qPCR or Next-Generation Sequencing (NGS), though in high-throughput workflows, this verification step is sometimes optional to maximize efficiency [1].
Test Phase
The Test phase serves as the empirical validation stage where experimentally measured performance data is collected for the engineered biological constructs [2]. This phase determines the efficacy of decisions made during the Design and Build phases through various functional assays tailored to the specific application. Testing can include measurements of protein expression levels, enzymatic activity, metabolic flux, growth characteristics, or other relevant phenotypic readouts [1]. The emergence of high-throughput screening technologies has dramatically accelerated this phase, allowing researchers to evaluate thousands of variants in parallel rather than individually. Advanced platforms can now incorporate analytical techniques such as size-exclusion chromatography (SEC) that simultaneously provide data on multiple protein characteristics including purity, yield, oligomeric state, and dispersity [4]. The reliability and throughput of testing methodologies directly impact the quality and quantity of data available for the subsequent Learn phase, making this stage crucial for the overall efficiency of the DBTL cycle.
Learn Phase
The Learn phase represents the analytical component of the cycle, where data collected during testing is processed and interpreted to extract meaningful insights. This stage involves comparing experimental results against the objectives established during the Design phase, identifying patterns, correlations, and causal relationships between design features and functional outcomes [2]. The knowledge generated during this phase informs the next iteration of design, creating a continuous improvement loop. Traditional Learning approaches relied heavily on researcher intuition and statistical analysis, but increasingly incorporate sophisticated computational tools and machine learning algorithms to uncover complex relationships within high-dimensional datasets [3]. The effectiveness of the Learn phase depends critically on both the quality of experimental data and the analytical frameworks employed to interpret it, ultimately determining how rapidly the DBTL cycle converges on optimal solutions.
Table 1: Key Stages of the Traditional DBTL Cycle
| Phase | Primary Activities | Outputs | Common Tools & Methods |
|---|---|---|---|
| Design | Define objectives; Select biological parts; Computational modeling | Genetic construct designs; Simulation predictions | CAD software; Bioinformatics databases; Metabolic models |
| Build | DNA synthesis; Vector assembly; Transformation | Physical DNA constructs; Engineered strains | Molecular cloning; DNA synthesis; Automated assembly; Sequencing |
| Test | Functional assays; Performance measurement; Data collection | Quantitative performance data; Expression levels; Activity metrics | HPLC; Spectroscopy; Chromatography; High-throughput screening |
| Learn | Data analysis; Pattern recognition; Hypothesis generation | Design rules; Predictive models; New research questions | Statistical analysis; Machine learning; Data visualization |
The Paradigm Shift: From DBTL to LDBT
The conventional DBTL cycle is undergoing a fundamental transformation driven by advances in machine learning and high-throughput experimental technologies. A groundbreaking paradigm shift, termed "LDBT" (Learn-Design-Build-Test), reorders the traditional cycle by placing Learning at the forefront [2] [3]. This approach leverages powerful machine learning models that interpret existing biological data to predict meaningful design parameters before any physical construction occurs [3]. The reordering addresses a critical limitation of the traditional DBTL cycle: the slow and resource-intensive nature of the Build-Test phases, which has historically created a bottleneck in biological design iterations [2].
The LDBT framework leverages the growing success of zero-shot predictions made by sophisticated AI models, where computational algorithms can generate functional biological designs without additional training or experimental data [2]. Protein language models—such as ESM and ProGen—trained on evolutionary relationships between millions of protein sequences have demonstrated remarkable capability in predicting beneficial mutations and inferring protein functions [2]. Similarly, structure-based deep learning tools like ProteinMPNN can design sequences that fold into specific backbone structures, leading to nearly a 10-fold increase in design success rates when combined with structure assessment tools like AlphaFold [2]. This paradigm shift brings synthetic biology closer to a "Design-Build-Work" model that relies on first principles, similar to disciplines like civil engineering, potentially reducing or eliminating the need for multiple iterative cycles [2].
Enabling Technologies Accelerating the Framework
Machine Learning and AI Integration
Machine learning has become a driving force in synthetic biology by enabling more efficient and scalable biological design. Unlike traditional biophysical models that are computationally expensive and limited in scope, machine learning methods can economically leverage large biological datasets to detect patterns in high-dimensional spaces [2]. Several specialized AI approaches have emerged for biological engineering:
Protein language models (e.g., ESM, ProGen) capture long-range evolutionary dependencies within amino acid sequences, enabling prediction of structure-function relationships [2]. These models have proven adept at zero-shot prediction of diverse antibody sequences and predicting solvent-exposed and charged amino acids [2].
Structure-based design tools (e.g., MutCompute, ProteinMPNN) use deep neural networks trained on protein structures to associate amino acids with their surrounding chemical environments, allowing prediction of stabilizing and functionally beneficial substitutions [2].
Functional prediction models focus on specific protein properties like thermostability and solubility. Tools such as Prethermut, Stability Oracle, and DeepSol predict effects of mutations on thermodynamic stability and solubility, helping researchers eliminate destabilizing mutations or identify stabilizing ones [2].
These machine learning approaches are increasingly being deployed in closed-loop design platforms where AI agents cycle through experiments autonomously, dramatically expanding capacity and reducing human intervention requirements [2].
Cell-Free Testing Platforms
Cell-free gene expression systems have emerged as a transformative technology for accelerating the Build and Test phases of the DBTL cycle. These platforms leverage protein biosynthesis machinery obtained from cell lysates or purified components to activate in vitro transcription and translation [2]. Their implementation offers several distinct advantages:
Exceptional speed: Cell-free systems can achieve protein production exceeding 1 g/L in less than 4 hours, dramatically faster than cellular expression systems [2].
Elimination of cloning steps: Synthesized DNA templates can be directly added to cell-free systems without intermediate, time-intensive cloning steps [2].
Tolerance to toxic products: Unlike living cells, cell-free systems enable production of proteins and pathways that would otherwise be toxic to host organisms [2].
Scalability and modularity: Reactions can be scaled from picoliters to kiloliters, and machinery can be obtained from organisms across the tree of life [2].
When combined with liquid handling robots and microfluidics, cell-free systems enable unprecedented throughput. For example, the DropAI platform leveraged droplet microfluidics and multi-channel fluorescent imaging to screen over 100,000 picoliter-scale reactions [2]. These capabilities make cell-free expression platforms particularly valuable for generating large-scale datasets needed to train machine learning models and validate computational predictions [2].
Automated Workflow Solutions
Automation technologies have become essential for implementing high-throughput DBTL cycles in practical research environments. Recent advancements focus on creating integrated systems that streamline the entire workflow from DNA to characterized protein:
The Semi-Automated Protein Production (SAPP) pipeline achieves a 48-hour turnaround from DNA to purified protein with only about six hours of hands-on time. This system uses miniaturized parallel processing in 96-well deep-well plates, auto-induction media, and two-step purification with parallel nickel-affinity and size-exclusion chromatography [4].
The DMX workflow addresses the DNA synthesis cost bottleneck by constructing sequence-verified clones from inexpensive oligo pools. This method uses an isothermal barcoding approach to tag gene variants within cell lysates, followed by long-read nanopore sequencing to link barcodes to full-length gene sequences, reducing per-design DNA construction costs by 5- to 8-fold [4].
Commercial systems like Nuclera's eProtein Discovery System unite design, expression, and purification in one connected workflow, enabling researchers to move from DNA to purified, soluble, and active protein in under 48 hours—a process that traditionally takes weeks [5].
These automated solutions share a common goal: replacing human variation with stable, reproducible systems that generate standardized, quantitative, high-quality experimental data at scales previously impractical [4] [5].
Table 2: Quantitative Performance Metrics of DBTL-Enabling Technologies
| Technology | Throughput Capacity | Time Reduction | Key Performance Metrics |
|---|---|---|---|
| Cell-Free Systems | >100,000 reactions via microfluidics [2] | Protein production in <4 hours vs. days/weeks [2] | >1 g/L protein yield; 48-hour DNA to protein [2] [5] |
| SAPP Workflow | 96 variants in one week [4] | 48-hour turnaround; 6 hours hands-on time [4] | ~90% cloning accuracy without sequencing [4] |
| DMX DNA Construction | 1,500 designs from single oligo pool [4] | 5-8 fold cost reduction [4] | 78% design recovery rate [4] |
| AI-Designed Proteins | 500,000+ computational surveys [2] | 10-fold increase in design success [2] | pM efficacy in neutralization assays [4] |
Experimental Protocols and Case Studies
High-Throughput Protein Engineering Protocol
The integration of machine learning with rapid experimental validation has enabled sophisticated protein engineering workflows. The following protocol outlines a representative approach for high-throughput protein characterization:
Computational Design: Generate initial protein variants using structure-based deep learning tools (e.g., ProteinMPNN) or protein language models (e.g., ESM). For structural motifs, combine sequence design with structure assessment using AlphaFold or RoseTTAFold to prioritize designs with high predicted stability [2].
DNA Library Construction: Convert digital designs to physical DNA using cost-effective methods. For large libraries (>100 variants), employ oligo pooling and barcoding strategies (e.g., DMX workflow) to reduce synthesis costs. For smaller libraries, utilize automated Golden Gate Assembly with suicide gene-containing vectors for high-fidelity, sequence-verification-free cloning [4].
Cell-Free Expression: Transfer DNA templates directly to cell-free transcription-translation systems arranged in 96- or 384-well formats. Utilize auto-induction media to eliminate manual induction steps. Incubate for 4-16 hours depending on protein size and yield requirements [2] [3].
Parallel Purification and Analysis: Perform two-step purification using nickel-affinity chromatography followed by size-exclusion chromatography (SEC) in deep-well plates. Use the SEC chromatograms to simultaneously assess protein purity, yield, oligomeric state, and dispersity [4].
Functional Characterization: Implement targeted assays based on protein function (e.g., fluorescence measurement, enzymatic activity, binding affinity). For high-throughput screening, leverage droplet microfluidics to analyze thousands of picoliter-scale reactions in parallel [2].
Data Integration and Model Retraining: Feed quantitative experimental results back into machine learning models to improve prediction accuracy for subsequent design rounds. Standardize data outputs using automated analysis tools to enable direct comparison between predicted and measured properties [4].
Case Study: AI-Driven Neutralizer Design
A compelling demonstration of the modern LDBT framework involved designing a potent neutralizer for Respiratory Syncytial Virus (RSV) [4]. Researchers began with a known binding protein (cb13) and fused it to 27 different oligomeric scaffolds to create a library of 58 multivalent constructs. Using the SAPP platform, they rapidly identified 19 correctly assembled and well-expressed multimers. Subsequent viral neutralization assays revealed that the best-performing dimer and trimer achieved IC50 values of 40 pM and 59 pM, respectively—a dramatic improvement over the monomer (5.4 nM) that surpassed the efficacy of MPE8 (156 pM), a leading commercial antibody targeting the same site [4]. This success highlighted a critical insight: the geometry of multimerization is crucial for function, and only a high-throughput platform makes it feasible to screen the vast combinatorial space required to discover optimal configurations.
Case Study: Antimicrobial Peptide Discovery
Researchers have successfully paired deep-learning sequence generation with cell-free expression to computationally survey over 500,000 antimicrobial peptides (AMP) and select 500 optimal variants for experimental validation [2]. This approach led to the identification of six promising AMP designs, demonstrating the power of machine learning to navigate vast sequence spaces and identify functional candidates with minimal experimental effort [2]. The combination of computational prescreening and rapid cell-free testing enabled comprehensive exploration of a sequence space that would be prohibitively large for conventional approaches.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Research Reagent Solutions for DBTL Implementation
| Reagent/Material | Function | Application Notes |
|---|---|---|
| Cell-Free TX-TL Systems | Provides transcription-translation machinery for protein synthesis without whole cells [2] | Enables rapid testing of genetic constructs; compatible with various source organisms; scalable from µL to L [2] |
| Golden Gate Assembly Mix | Modular DNA assembly using Type IIS restriction enzymes [4] | Achieves ~90% cloning accuracy; enables sequencing-free cloning when combined with ccdB suicide gene vectors [4] |
| Oligo Pools | Cost-effective DNA library synthesis [4] | DMX workflow reduces cost 5-8 fold; enables construction of thousands of variants from single pool [4] |
| Auto-Induction Media | Automates protein expression induction [4] | Eliminates manual induction step in high-throughput workflows; compatible with deep-well plate formats [4] |
| Nickel-Affinity Resin | Purification of histidine-tagged proteins [4] | Compatible with miniaturized formats; first step in two-step purification process [4] |
| Size-Exclusion Chromatography Plates | High-throughput protein analysis and purification [4] | Simultaneously assesses purity, yield, oligomeric state, and dispersity [4] |
Workflow Visualization
LDBT Cycle: AI-First Biological Design
Cell-Free Protein Production Workflow
The DBTL framework continues to evolve from a conceptual model to an engineering reality that increasingly relies on the integration of computational and experimental technologies. The emergence of the LDBT paradigm represents a fundamental shift toward data-driven biological design, where machine learning models pre-optimize designs in silico before physical implementation [2] [3]. This approach is made possible by the growing success of zero-shot prediction methods and the availability of large biological datasets for training sophisticated AI models [2].
Looking forward, the convergence of several technological trends promises to further accelerate biological engineering. The integration of multi-omics datasets—transcriptomics, proteomics, and metabolomics—into the LDBT framework will enhance machine learning models' breadth and precision, capturing not only static sequence features but dynamic cellular contexts [3]. Advances in automation and microfluidics will continue to push throughput boundaries while reducing costs and hands-on time [2] [5]. Perhaps most significantly, the development of fully autonomous self-driving laboratories represents the ultimate expression of the DBTL cycle, where AI systems design, execute, and interpret experiments with minimal human intervention [4].
As these technologies mature, the DBTL framework will increasingly support a future where biological engineering becomes more predictable, scalable, and accessible. This progression promises to democratize synthetic biology research, enabling smaller labs and startups to participate in cutting-edge bioengineering without requiring extensive infrastructure [3]. By continuing to refine and implement the DBTL framework—in both its traditional and reimagined forms—the research community can accelerate the development of novel biologics, sustainable bioprocesses, and advanced biomaterials that address pressing challenges in healthcare, energy, and environmental sustainability.
The engineering of biological systems has undergone a fundamental transformation with the adoption of the Design-Build-Test-Learn (DBTL) cycle, which has largely supplanted traditional linear development approaches. This iterative framework provides a systematic methodology for optimizing genetic constructs, metabolic pathways, and cellular functions through rapid experimentation and data-driven learning. By embracing DBTL cycles, synthetic biologists have dramatically accelerated the development of engineered biological systems for applications ranging from pharmaceutical production to sustainable manufacturing. This technical guide examines the core principles of the DBTL framework, its implementation across diverse synthetic biology applications, and the emerging technologies that are further enhancing its efficiency and predictive power.
Traditional linear approaches to biological engineering followed a sequential "design-build-test" pattern without structured iteration, making the process slow, inefficient, and often unreliable [6]. Each genetic design required complete development before testing, with no formal mechanism for incorporating insights from failures into improved designs. This limitation became particularly problematic in complex biological systems where unpredictable interactions between components frequently occurred.
The DBTL framework emerged as a solution to these challenges by introducing a structured, cyclical process for engineering biological systems [1]. This iterative approach enables synthetic biologists to systematically explore design spaces, test hypotheses, and incrementally improve system performance through successive cycles of refinement. The paradigm shift from linear to iterative development has fundamentally transformed synthetic biology, enabling more predictable engineering of biological systems and reducing development timelines from years to months in many applications.
The Core DBTL Cycle: Components and Implementation
Phase 1: Design
The Design phase involves creating genetic blueprints based on biological knowledge and engineering objectives. Researchers define specifications for desired biological functions and design genetic parts or systems accordingly, which may include introducing novel components or redesigning existing parts for new applications [2]. This phase relies on domain expertise, computational modeling, and increasingly on predictive algorithms.
Key Design Tools and Approaches:
- Retrosynthesis Software: Tools like RetroPath2.0 identify metabolic pathways linking target compounds to host metabolites [7]
- Standardized Biological Parts: Modular DNA components with characterized functions enable predictable system design
- Pathway Enumeration Algorithms: Computational methods generate multiple pathway alternatives for testing
- Machine Learning-Guided Design: Models trained on biological data predict part performance before physical construction [8]
Phase 2: Build
The Build phase translates digital designs into physical biological constructs. DNA sequences are synthesized or assembled into plasmids or other vectors, then introduced into host systems such as bacteria, yeast, or cell-free expression platforms [2]. Automation and standardization have dramatically increased the throughput and reliability of this phase.
Build Technologies and Methods:
- DNA Synthesis Services: Companies like Synbio Technologies and Twist Bioscience provide custom DNA library synthesis with high diversity (up to 10^10 variants) [6] [9]
- Automated Strain Engineering: Robotic systems enable high-throughput genetic modification
- Standardized Assembly Protocols: Methods like Golden Gate, Gibson Assembly, and Ligation Chain Reaction (LCR) enable modular construction [7]
- Cell-Free Systems: Cell-free gene expression using purified cellular machinery accelerates building and testing [2]
Phase 3: Test
The Test phase experimentally characterizes the performance of built constructs against design objectives. This involves measuring relevant phenotypic properties, production yields, functional activities, or other system behaviors using appropriate analytical methods [2] [1].
Testing Methodologies and Platforms:
- High-Throughput Screening: Automated systems rapidly assay thousands of variants
- Multi-Omics Analyses: Transcriptomics, proteomics, and metabolomics provide comprehensive system characterization
- Cell-Free Testing: In vitro expression systems enable rapid functional analysis without cellular constraints [2]
- Analytical Chemistry: HPLC, MS, and fluorescence-based assays quantify product formation and kinetics
Phase 4: Learn
The Learn phase analyzes experimental data to extract insights that inform subsequent design cycles. Researchers identify patterns, correlations, and causal relationships between genetic designs and observed functions, creating knowledge that improves prediction accuracy in future iterations [1].
Learning Approaches:
- Statistical Analysis: Identifies significant correlations between design parameters and outcomes
- Machine Learning: Algorithms learn complex sequence-function relationships from experimental data [8]
- Mechanistic Modeling: Kinetic models interpret results based on biological principles [10]
- Pathway Analysis: Tools like rpThermo and rpFBA evaluate pathway performance based on thermodynamics and flux balance [7]
DBTL in Action: Experimental Applications and Protocols
Case Study 1: Optimizing Dopamine Production in E. coli
A knowledge-driven DBTL approach was applied to develop an efficient dopamine production strain in E. coli, resulting in a 2.6 to 6.6-fold improvement over previous methods [11].
Experimental Protocol:
- Strain Engineering:
- Host strain E. coli FUS4.T2 was engineered for high L-tyrosine production by deleting the tyrosine repressor TyrR and mutating feedback inhibition in chorismate mutase/prephenate dehydrogenase (tyrA)
- Heterologous genes hpaBC (encoding 4-hydroxyphenylacetate 3-monooxygenase) and ddc (encoding L-DOPA decarboxylase) were introduced via pET plasmid system
In Vitro Pathway Testing:
- Cell-free protein synthesis systems using crude cell lysates tested different relative enzyme expression levels
- Reaction buffer contained 0.2 mM FeCl₂, 50 μM vitamin B₆, and 1 mM L-tyrosine or 5 mM L-DOPA in 50 mM phosphate buffer (pH 7)
In Vivo Optimization:
- RBS engineering fine-tuned translation initiation rates for hpaBC and ddc genes
- 5 mL culture volumes in minimal medium (20 g/L glucose, 10% 2xTY, MOPS buffer) were used in high-throughput screening
- Dopamine production was quantified via HPLC analysis
Results and Iteration:
- Initial designs produced 27 mg/L dopamine
- After three DBTL cycles incorporating RBS optimization insights, production increased to 69.03 ± 1.2 mg/L (34.34 ± 0.59 mg/g biomass)
Case Study 2: RNA Toehold Switch Optimization
The DBTL cycle was applied across 10 iterations to optimize RNA toehold switches for diagnostic applications, demonstrating rapid performance improvement through structured iteration [12].
Experimental Protocol:
- Initial Design (Trial 1):
- Toehold switch designed to activate amilCP chromoprotein reporter upon target RNA binding
- DNA templates resuspended at 160 nM for consistent cell-free expression composition
Iterative Refinement (Trials 2-5):
- Trial 2: Replaced amilCP with GFP for improved kinetic measurements, revealing OFF-state leakiness
- Trial 3: Added upstream buffer sequences to stabilize the OFF state, reducing leak but limiting ON-state expression
- Trial 4: Reduced downstream guanine content to minimize ribosomal stalling
- Trial 5: Implemented superfolder GFP (sfGFP) for faster maturation and brighter signal
Validation (Trials 6-10):
- Conducted reproducibility testing across biological replicates
- Measured fluorescence kinetics and fold-activation ratios
- Final design achieved 2.0x fold-activation with high statistical significance (p = 1.43 × 10⁻¹¹¹)
Key Learning Outcomes:
- Reporter protein selection critically impacts measurement sensitivity
- Upstream sequences influence switch leakiness
- Downstream sequence composition affects translational efficiency
- sfGFP provides optimal balance of speed, brightness, and reliability for toehold switches
Quantitative Performance Comparison: DBTL vs. Linear Approaches
Table 1: Performance Metrics Comparing DBTL and Linear Development Approaches
| Development Metric | Traditional Linear Approach | DBTL Cycle Approach | Improvement Factor |
|---|---|---|---|
| Development Timeline | 12-24 months | 3-6 months | 4x faster |
| Experimental Throughput | 10-100 variants/cycle | 1,000-100,000 variants/cycle | 100-1000x higher |
| Success Rate | 5-15% | 25-50% | 3-5x higher |
| Data Generation | Limited, unstructured | Comprehensive, structured | 10-100x more data |
| Resource Efficiency | High waste, repeated efforts | Optimized, iterative learning | 2-3x more efficient |
Table 2: DBTL Performance in Published Case Studies
| Application | Number of DBTL Cycles | Initial Performance | Final Performance | Key Optimized Parameters |
|---|---|---|---|---|
| Dopamine Production [11] | 3 | 27 mg/L | 69.03 ± 1.2 mg/L | RBS strength, enzyme expression ratio |
| Toehold Switches [12] | 10 | High leak, low activation | 2.0x fold activation, minimal leak | Reporter choice, UTR sequences |
| Lycopene Production [7] | 4 | 0.5 mg/g DCW | 15.2 mg/g DCW | Promoter strength, enzyme variants |
| PET Hydrolase [2] | 2 | Low stability | Increased stability & activity | Protein sequence, stabilizing mutations |
The Scientist's Toolkit: Essential Research Reagents and Solutions
Table 3: Key Research Reagents and Solutions for DBTL Implementation
| Reagent/Solution | Function | Example Applications | Implementation Notes |
|---|---|---|---|
| DNA Library Synthesis Services | Generate variant libraries for screening | Protein engineering, pathway optimization | Twist Bioscience, GENEWIZ offer diversity up to 10^12 variants [13] [9] |
| Cell-Free Expression Systems | Rapid in vitro testing of genetic designs | Protein production, circuit characterization | >1 g/L protein in <4 hours; pL to kL scalability [2] |
| Automated Strain Engineering Platforms | High-throughput genetic modification | Metabolic engineering, host optimization | Biofoundries enable construction of 1,000+ strains/week [7] |
| Analytical Screening Tools | Quantify strain performance | Metabolite production, growth assays | HPLC, MS, fluorescence enable high-throughput phenotyping [11] |
| Machine Learning Algorithms | Predictive design from experimental data | Protein engineering, pathway optimization | Gradient boosting, random forest effective in low-data regimes [10] [8] |
| Standardized Genetic Parts | Modular, characterized DNA elements | Genetic circuit design, metabolic pathway assembly | Registry of Standard Biological Parts enables predictable engineering |
Advanced DBTL: Integrating Machine Learning and Automation
The Rise of Machine Learning in DBTL Cycles
Machine learning (ML) has transformed the Learn and Design phases of DBTL cycles by enabling predictive modeling from complex biological data. ML algorithms can identify non-intuitive patterns in high-dimensional biological data, dramatically accelerating the design process [8].
Key ML Applications in DBTL:
- Protein Engineering: Models like ProteinMPNN and ESM predict protein structures and functions from sequences [2]
- Pathway Optimization: Gradient boosting and random forest models predict optimal enzyme expression levels and identify rate-limiting steps [10]
- Experimental Design: Active learning algorithms prioritize the most informative experiments for each cycle
- Automated Recommendation: Systems suggest improved designs based on previous cycle results [10]
The LDBT Paradigm Shift: Learning Before Design
A significant evolution in the DBTL framework is the emergence of the LDBT (Learn-Design-Build-Test) paradigm, where machine learning precedes initial design [2]. This approach leverages pre-trained models on large biological datasets to make zero-shot predictions, potentially eliminating multiple DBTL cycles.
LDBT Enabling Technologies:
- Protein Language Models: ESM and ProGen capture evolutionary relationships to predict protein functions [2]
- Structure-Based Design: AlphaFold and RoseTTAFold enable structure-based protein engineering
- Foundational Models: Large-scale models trained on diverse biological data generalize across tasks
- Hybrid Modeling: Physics-informed machine learning combines statistical power with mechanistic principles [2]
Automated Workflow Platforms
Integrated platforms like Galaxy-SynBioCAD provide end-to-end workflow automation for DBTL implementation [7]. These systems connect pathway design, DNA assembly planning, and experimental execution through standardized data formats (SBML, SBOL) and automated liquid handling integration.
Visualization of DBTL Workflows and Relationships
The Core DBTL Cycle
Diagram 1: Core DBTL Cycle - The iterative engineering framework showing the four phases and their relationships.
The Emerging LDBT Paradigm
Diagram 2: LDBT Paradigm - The emerging framework where machine learning precedes design, potentially reducing iteration needs.
The DBTL cycle has fundamentally transformed synthetic biology by replacing inefficient linear development with a structured, iterative approach that embraces biological complexity. Through successive rounds of refinement, synthetic biologists can now engineer biological systems with unprecedented efficiency and predictability. The integration of machine learning, automation, and cell-free technologies continues to accelerate DBTL cycles, while the emerging LDBT paradigm promises to further reduce development timelines by leveraging predictive modeling before physical construction. As these methodologies mature, DBTL-based approaches will continue to drive innovations across biotechnology, from sustainable manufacturing to therapeutic development, solidifying their role as the cornerstone of modern biological engineering.
The Design-Build-Test-Learn (DBTL) cycle serves as the fundamental engineering framework in synthetic biology, providing a systematic and iterative methodology for developing and optimizing biological systems. This disciplined approach enables researchers to engineer microorganisms for specific functions, such as producing fine chemicals, pharmaceuticals, and biofuels [1]. The DBTL cycle's power lies in its iterative nature—complex biological engineering projects rarely succeed on the first attempt but instead make continuous progress through sequential cycles of refinement and improvement [14]. As the field advances, emerging technologies like machine learning and cell-free systems are reshaping the traditional DBTL paradigm, potentially reordering the cycle itself to accelerate biological design [2]. This technical guide examines the core components, interdependencies, and evolving methodologies of the DBTL framework within modern biological engineering research.
The DBTL Cycle: Core Components and Interdependencies
Design Phase
The Design phase initiates the DBTL cycle by establishing clear objectives and creating rational plans for biological system engineering. This stage relies on domain knowledge, expertise, and computational modeling tools to define specifications for genetic parts and systems [2]. Researchers design genetic constructs by selecting appropriate biological components such as promoters, ribosomal binding sites (RBS), and coding sequences, then assembling them into functional circuits or metabolic pathways using standardized methods [14].
Advanced biofoundries employ integrated software suites for automated pathway and enzyme selection. Tools like RetroPath and Selenzyme enable in silico selection of candidate enzymes and pathway designs, while PartsGenie facilitates the design of reusable DNA parts with simultaneous optimization of bespoke ribosome-binding sites and enzyme coding regions [15]. These components are combined into combinatorial libraries of pathway designs, which are statistically reduced using design of experiments (DoE) methodologies to create tractable numbers of samples for laboratory construction [15]. The transition from purely rational design to data-driven approaches represents a significant shift in synthetic biology, with machine learning models increasingly informing the design process based on prior knowledge [2].
Build Phase
The Build phase translates theoretical designs into physical biological reality through molecular biology techniques. This hands-on stage involves DNA synthesis, plasmid cloning, and transformation of engineered constructs into host organisms [14]. Automation has become crucial in this phase, with robotic platforms performing assembly techniques like ligase cycling reaction (LCR) to construct pathway variants [15].
High-throughput building processes enable the creation of diverse biological libraries. For example, in metabolic pathway optimization, researchers vary enzyme levels through promoter engineering or RBS modifications to create numerous strain designs [10]. After assembly, constructs undergo quality control through automated purification, restriction digest analysis via capillary electrophoresis, and sequence verification [15]. The modular nature of modern building approaches allows researchers to efficiently test multiple permutations by interchanging standardized biological components, significantly accelerating strain development [1].
Test Phase
The Test phase focuses on robust data collection through quantitative measurements of engineered system performance. Researchers employ various assays to characterize biological behavior, including measuring fluorescence to quantify gene expression, performing microscopy to observe cellular changes, and conducting biochemical assays to analyze metabolic pathway outputs [14].
Advanced testing methodologies incorporate high-throughput screening in multi-well plates combined with analytical techniques like ultra-performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS) for precise quantification of target compounds and intermediates [15]. Testing also extends to bioprocess performance evaluation, where parameters such as biomass growth, substrate consumption, and product formation are monitored over time [10]. The emergence of cell-free expression systems has dramatically accelerated testing by enabling rapid in vitro protein synthesis and functional characterization without time-intensive cloning steps [2]. These systems leverage protein biosynthesis machinery from cell lysates for in vitro transcription and translation, allowing high-throughput sequence-to-function mapping of protein variants [2].
Learn Phase
The Learn phase represents the analytical core of the cycle, where experimental data transforms into actionable knowledge. Researchers analyze and interpret test results to determine whether designs functioned as expected and identify underlying principles governing system behavior [14]. This stage employs statistical methods and machine learning algorithms to identify relationships between design factors and observed performance metrics [15].
In metabolic engineering, the learning process often involves using kinetic modeling frameworks to understand pathway behavior and identify optimization targets [10]. The insights gained—whether from success or failure—directly inform the subsequent Design phase, leading to improved hypotheses and refined designs [14]. As machine learning advances, the learning phase has begun to shift earlier in the cycle, with some proposals suggesting an "LDBT" approach where learning precedes design through zero-shot predictions from pre-trained models [2].
Table 1: Key Tools and Technologies Enhancing DBTL Cycles
| DBTL Phase | Tools & Technologies | Applications | Impact |
|---|---|---|---|
| Design | RetroPath, Selenzyme, PartsGenie, ProteinMPNN, ESM | Pathway design, enzyme selection, part optimization, protein engineering | Accelerates in silico design; enables zero-shot predictions |
| Build | Automated DNA assembly, ligase cycling reaction (LCR), robotic platforms | High-throughput construct assembly, library generation | Increases throughput; reduces manual labor and human error |
| Test | UPLC-MS/MS, cell-free systems, droplet microfluidics, fluorescent assays | Metabolite quantification, rapid prototyping, ultra-high-throughput screening | Enables megascale data generation; accelerates characterization |
| Learn | Machine learning (gradient boosting, random forest), kinetic modeling, statistical analysis | Data pattern recognition, predictive modeling, design recommendation | Identifies non-intuitive relationships; guides next-cycle designs |
Advanced Methodologies: Machine Learning and Automation
Machine learning has revolutionized the DBTL cycle by enabling data-driven biological design. Supervised learning models, particularly gradient boosting and random forest algorithms, have demonstrated strong performance in the low-data regimes common in biological engineering [10]. These models can predict strain performance based on genetic designs, allowing researchers to prioritize the most promising candidates for experimental testing.
Protein language models like ESM and ProGen, trained on evolutionary relationships between millions of protein sequences, enable zero-shot prediction of protein functions and beneficial mutations [2]. Structure-based deep learning tools such as ProteinMPNN facilitate protein design by predicting sequences that fold into desired backbone structures, achieving nearly 10-fold increases in design success rates when combined with structure assessment tools like AlphaFold [2]. These capabilities are transforming the DBTL paradigm from empirical iteration toward predictive engineering.
Automation represents another critical advancement, with biofoundries implementing end-to-end automated DBTL pipelines. These integrated systems handle pathway design, DNA assembly, strain construction, performance testing, and data analysis with minimal manual intervention [15]. The modular nature of these pipelines allows customization for different host organisms and target compounds while maintaining efficient workflow and iterative optimization capabilities.
Diagram 1: Traditional DBTL Cycle Workflow
Case Studies in Biological Engineering
Metabolic Pathway Optimization for Fine Chemical Production
An integrated DBTL pipeline successfully optimized (2S)-pinocembrin production in E. coli, achieving a 500-fold improvement through two iterative cycles [15]. The initial design created 2,592 possible pathway configurations through combinatorial variation of vector copy number, promoter strength, and gene order. Statistical reduction via design of experiments condensed this to 16 representative constructs. Testing revealed vector copy number as the most significant factor affecting production, followed by chalcone isomerase promoter strength. The learning phase informed a second design round focusing on high-copy-number vectors with optimized gene positioning, ultimately achieving competitive titers of 88 mg/L [15].
Development of Dopamine Production Strains
A knowledge-driven DBTL approach developed an efficient dopamine production strain in E. coli [11]. Researchers incorporated upstream in vitro investigation using cell-free protein synthesis systems to test different enzyme expression levels before DBTL cycling. This knowledge-informed design was then translated to an in vivo environment through high-throughput RBS engineering. The optimized strain achieved dopamine production of 69.03 ± 1.2 mg/L (34.34 ± 0.59 mg/gbiomass), representing a 2.6 to 6.6-fold improvement over previous state-of-the-art production systems [11].
Anti-adipogenic Protein Discovery
A systematic DBTL approach identified a novel anti-adipogenic protein from Lactobacillus rhamnosus [14]. The first cycle tested the hypothesis that direct bacterial contact inhibits adipogenesis by co-culturing six Lactobacillus strains with 3T3-L1 preadipocytes. Results showed 20-30% inhibition of lipid accumulation, confirming anti-adipogenic effects. The second cycle investigated whether secreted extracellular substances mediated this effect by testing bacterial supernatants, revealing that only L. rhamnosus supernatant produced concentration-dependent inhibition (up to 45%). The third cycle isolated exosomes from supernatants, demonstrating that L. rhamnosus exosomes reduced lipid accumulation by 80% through regulation of PPARγ, C/EBPα, and AMPK pathways [14].
Table 2: Quantitative Results from DBTL Case Studies
| Case Study | Initial Performance | Optimized Performance | Key Optimization Factors | DBTL Cycles |
|---|---|---|---|---|
| Pinocembrin Production [15] | 0.002 - 0.14 mg/L | 88 mg/L (500x improvement) | Vector copy number, CHI promoter strength | 2 |
| Dopamine Production [11] | Baseline (state-of-the-art) | 69.03 mg/L (2.6-6.6x improvement) | RBS engineering, GC content in SD sequence | Multiple with in vitro pre-screening |
| Anti-adipogenic Discovery [14] | 20-30% lipid reduction (raw bacteria) | 80% lipid reduction (exosomes) | Identification of active component, AMPK pathway regulation | 3 |
Experimental Protocols and Methodologies
Cell-Free Protein Synthesis for Rapid Testing
Cell-free expression systems provide a powerful platform for rapid DBTL cycling [2]. These systems leverage protein biosynthesis machinery from crude cell lysates or purified components to activate in vitro transcription and translation. The standard protocol involves:
Lysate Preparation: Cultivate source organisms (e.g., E. coli), harvest cells during exponential growth, and lyse using French press or sonication. Clarify lysates by centrifugation.
Reaction Assembly: Combine DNA templates with cell-free reaction mixtures containing amino acids, energy sources (ATP, GTP), energy regeneration systems, and cofactors.
Protein Synthesis: Incubate reactions at 30-37°C for 4-6 hours, achieving protein yields exceeding 1 g/L [2].
Functional Analysis: Test synthesized proteins directly in coupled colorimetric or fluorescent-based assays for high-throughput sequence-to-function mapping.
This approach enables testing without molecular cloning or transformation steps, dramatically accelerating the Build-Test phases [2].
High-Throughput RBS Engineering
Ribosome Binding Site engineering enables precise fine-tuning of gene expression in synthetic pathways [11]. The methodology includes:
Library Design: Design RBS variants with modulated Shine-Dalgarno sequences while maintaining surrounding sequences to avoid secondary structure changes.
Library Construction: Assemble RBS variants via PCR-based methods or automated DNA assembly using robotic platforms.
Host Transformation: Introduce variant libraries into production hosts (e.g., E. coli FUS4.T2 for dopamine production).
Screening: Culture transformants in 96-deepwell plates with automated media handling and induction protocols.
Product Quantification: Analyze culture supernatants using UPLC-MS/MS for precise metabolite measurement.
Data Analysis: Correlate RBS sequences with production levels to identify optimal expression configurations.
Kinetic Modeling for Metabolic Pathway Optimization
Mechanistic kinetic models simulate metabolic pathway behavior to guide DBTL cycles [10]. The implementation involves:
Model Construction: Develop ordinary differential equations describing intracellular metabolite concentration changes, with reaction fluxes based on kinetic mechanisms derived from mass action principles.
Parameterization: Incorporate kinetic parameters from literature or experimental measurements, verifying physiological relevance through tools like ORACLE sampling.
Virtual Screening: Simulate pathway performance across combinatorial libraries of enzyme expression levels by adjusting Vmax parameters.
Machine Learning Integration: Use simulation data to train and benchmark machine learning models (e.g., gradient boosting, random forest) for predicting optimal pathway configurations.
Experimental Validation: Test model-predicted optimal designs and iteratively refine models based on experimental discrepancies.
Essential Research Reagent Solutions
Table 3: Key Research Reagents for DBTL Workflows
| Reagent/Resource | Function | Application Examples |
|---|---|---|
| Cell-Free Systems | In vitro transcription/translation | Rapid protein synthesis, pathway prototyping [2] |
| DNA Assembly Kits | Modular construction of genetic circuits | Golden Gate assembly, ligase cycling reaction [15] |
| RBS Libraries | Fine-tuning gene expression | Metabolic pathway optimization, enzyme balancing [11] |
| Promoter Collections | Transcriptional regulation | Combinatorial pathway optimization [10] |
| Analytical Standards | Metabolite quantification | UPLC-MS/MS calibration, product verification [15] |
| Specialized Media | Selective cultivation | High-throughput screening, production optimization [11] |
The Evolving DBTL Paradigm: LDBT and Future Directions
The traditional DBTL cycle is evolving toward an LDBT paradigm, where Learning precedes Design through machine learning [2]. This approach leverages pre-trained models on large biological datasets to make zero-shot predictions for biological design, potentially reducing or eliminating iterative cycling. Advances in protein language models (ESM, ProGen) and structure-based design tools (ProteinMPNN, MutCompute) enable increasingly accurate computational predictions of protein structure and function [2].
Cell-free platforms continue to accelerate Build-Test phases, with droplet microfluidics enabling screening of >100,000 reactions [2]. Integration of these technologies with automated biofoundries creates continuous DBTL pipelines that systematically address biological design challenges. As these capabilities mature, synthetic biology moves closer to a Design-Build-Work model based on first principles, similar to established engineering disciplines [2].
Diagram 2: Emerging LDBT (Learn-Design-Build-Test) Paradigm
The DBTL cycle remains the cornerstone methodology for synthetic biology and biological engineering, providing a disciplined framework for tackling biological design challenges. The interdependent phases create a powerful iterative process where each cycle builds upon knowledge gained from previous iterations. As machine learning, automation, and cell-free technologies continue to advance, the DBTL paradigm is evolving toward more predictive and efficient engineering approaches. For researchers in drug development and biological engineering, mastering DBTL principles and methodologies provides a critical foundation for success in developing novel biological solutions to complex challenges.
In the context of the Design-Build-Test-Learn (DBTL) cycle for biological engineering research, the "Learn" phase represents a critical juncture where experimental data is transformed into actionable knowledge to inform the next cycle of design. This phase has historically constituted a significant bottleneck in research and drug development. The challenge has not been a scarcity of data; rather, it has been the computational and analytical struggle to extract meaningful, causal insights from the enormous volumes and complexity of biological and clinical data generated in the "Test" phase. The transition from high-throughput experimental data to reliable biological knowledge has been hampered by issues of data quality, integration, standardization, and the inherent limitations of analytical models, often causing costly delays and reducing the overall efficiency of the DBTL cycle [16] [4].
This article explores the historical and technical dimensions of this "Learn" bottleneck, framing the discussion within the broader thesis of the DBTL cycle's role in advancing biological engineering. For researchers, scientists, and drug development professionals, overcoming this bottleneck is paramount to accelerating the discovery of novel therapeutics, optimizing bioprocesses, and realizing the full potential of personalized medicine.
The Data Deluge in Biological Engineering
The "Test" phase of the DBTL cycle generates data at a scale that has overwhelmed traditional analytical approaches. The diversity and volume of this data are immense, originating from a wide array of high-throughput technologies.
Table 1: Common Types of Big Data in Biological Engineering
| Data Type | Description | Examples & Sources |
|---|---|---|
| Genomic Data | Information about an organism's complete set of DNA, including genes and non-coding sequences. | European Nucleotide Archive (ENA) [17]; Genomic sequencing data from bacteriophage ϕX174 to the 160 billion base pairs of Tmesipteris oblanceolata [17]. |
| Clinical Trial Data | Structured and unstructured data collected during clinical trials to evaluate drug safety and efficacy. | Protocols, demographic data, outcomes, and adverse event reports [16]. |
| Real-World Evidence (RWE) | Data derived from real-world settings outside of traditional clinical trials. | Electronic Health Records (EHRs), claims data, wearables, and patient surveys [16] [18]. |
| Proteomic & Metabolomic Data | Data on the entire set of proteins (proteome) or small-molecule metabolites (metabolome) in a biological system. | Mass spectrometry data; multi-omics datasets for holistic biological analysis [16] [17]. |
| Pharmacovigilance Data | Data related to the detection, assessment, and prevention of adverse drug effects. | FDA's Adverse Events Reporting System (FAERS), Vigibase, social media posts [16] [18]. |
| Imaging Data | Radiology scans and diagnostic imagery. | Data analyzed with AI for early detection and treatment optimization [16]. |
Table 2: Scale of Biological Data Repositories (Representative Examples)
| Repository/Database | Primary Content | Reported Scale |
|---|---|---|
| EMBL Data Resources | A collection of biological data resources. | Approximately 100 petabytes (10^15 bytes) of raw biological data across 54 resources [17]. |
| Database Commons | A curated catalog of biological databases. | 5,825 biological databases (as of 2023, increased 19.2% since) covering 1,728 species [17]. |
The sheer volume and heterogeneity of these datasets present the initial hurdle for the "Learn" phase. Integrating clinical trial results, genomic sequencing, EHRs, and post-market surveillance data requires extensive data mapping and harmonization to achieve a consistent and analyzable dataset [16]. Furthermore, the quality of the input data directly determines the reliability of the output knowledge. Duplicates, missing fields, and inconsistent units can severely distort analytical outcomes, making proactive validation pipelines and anomaly detection systems essential [16].
Historical & Technical Challenges in the 'Learn' Phase
The process of learning from big data is fraught with technical challenges that have historically slowed research progress. These challenges extend beyond simple data volume to the very structure, quality, and context of the data.
Data Integration and Standardization
A primary challenge is the fragmented nature of data sources. EHRs, laboratory instruments, and various 'omics platforms all produce data in different formats and structures (structured, semi-structured, and unstructured) [16]. Integrating these for a unified analysis is a non-trivial task that requires extensive computational and human resources. As noted in the context of pharmaceutical research, achieving interoperability between these disparate systems is a fundamental prerequisite for any meaningful learning [16].
Data Accuracy, Quality, and Context
The analytical outputs of the "Learn" phase are only as good as the input data. The principle of "garbage in, garbage out" is acutely relevant here. Key issues include:
- Data Incompleteness: In EHRs, data can be missing due to patients transferring between healthcare systems or inconsistent documentation of over-the-counter medications [18].
- Reporting Bias: In spontaneous reporting systems like FAERS, reports are voluntary, which can lead to underreporting or overreporting of certain events based on factors like a drug's time on the market or media coverage [18].
- Loss of Context: The conversion of unstructured data (e.g., free-text physician notes) into structured data for analysis can lead to a loss of nuanced information [18].
Analytical and Interpretive Limitations
Even with clean, integrated data, the analytical methods themselves present challenges.
- Distinguishing Causation from Correlation: A significant challenge in data mining clinical records is distinguishing causal relationships from mere associations. For example, an analysis might find an association between a gadolinium-based contrast agent and myopathy. However, this is more likely because the agent was used to diagnose the condition, not cause it, highlighting the risk of misinterpretation without clinical expertise [18].
- The "Gold Standard" Problem: In drug-drug interaction (DDI) studies, it is notoriously difficult to define "true positive" and "true negative" interactions. The lack of a definitive standard resource for DDIs, combined with clinical prescribing biases that avoid known risky combinations, makes it hard to validate findings from data mining approaches [18].
- Model Precision and "Microbial Dark Matter": In environmental biotechnology, a vast proportion of genes identified in metagenomic datasets encode proteins of unknown function. This "microbial dark matter" limits our ability to reconstruct complete metabolic pathways, such as those for PFAS degradation, from omics data alone [17].
Case Studies & Experimental Protocols
The historical challenges of the "Learn" bottleneck can be clearly illustrated through specific experimental workflows in drug development and synthetic biology.
Case Study 1: Post-Marketing Drug Safety Surveillance
Objective: To identify novel drug-drug interactions (DDIs) leading to adverse drug events (ADEs) after a drug has been released to the market using large-scale healthcare data.
Methodology:
- Data Acquisition: Gather data from sources such as:
- Spontaneous Reporting Systems (SRS): FDA's FAERS or WHO's Vigibase.
- Electronic Health Records (EHRs): Structured data (lab results, diagnosis codes) and unstructured data (clinical notes).
- Healthcare Claims Data: Structured data on pharmacy and medical claims [18].
- Data Preprocessing and Harmonization: Convert unstructured data to structured format using Natural Language Processing (NLP). Map different coding systems to a standard vocabulary. This step is where significant information loss can occur [18].
- Data Mining and Analysis: Apply statistical and machine learning algorithms (e.g., disproportionality analysis in SRS, regression models in EHRs) to detect signals of association between drug pairs and adverse events [18].
- Signal Validation and Triangulation:
- Clinical Expert Review: A clinician assesses the plausibility of the detected signal.
- Mechanistic Investigation: Conduct in vitro studies (e.g., cytochrome P450 inhibition assays) to explore a biological mechanism.
- Pathway Analysis: Integrate drug-gene interaction data to build drug-gene-drug networks that support the finding [18].
Historical Bottleneck: The "Learn" phase here is hampered by reporting biases, the difficulty of defining true negative DDIs, and the fundamental challenge of establishing causation from observational data. These limitations mean that findings are often hypothesis-generating rather than conclusive, requiring further costly and time-consuming experimental validation [18].
Case Study 2: High-Throughput Protein Engineering
Objective: To rapidly design, produce, and characterize thousands of protein variants and use the resulting data to improve AI models for the next design cycle—closing the DBTL loop.
Experimental Protocol (SAPP/DMX Platform): This protocol was developed to address the bottleneck that occurs when AI can design proteins faster than they can be physically tested [4].
- Design: AI models (e.g., RFdiffusion, ProteinMPNN) generate digital blueprints for novel proteins.
- Build:
- DNA Construction (DMX Workflow): Use inexpensive oligo pools. Employ an isothermal barcoding method to tag each gene variant within a cell lysate. Use long-read nanopore sequencing to link each barcode to its full-length gene sequence. This reduces DNA synthesis costs by 5- to 8-fold [4].
- Cloning (SAPP Workflow): Use Golden Gate Assembly with a vector containing a "suicide gene" (ccdB) to achieve ~90% cloning accuracy, eliminating the need for sequencing [4].
- Protein Production (SAPP Workflow): Conduct expression and purification in 96-well deep-well plates using auto-induction media and a two-step parallel purification (nickel-affinity and size-exclusion chromatography).
- Test:
- Size-Exclusion Chromatography (SEC): The SEC step simultaneously provides high-throughput data on protein purity, yield, oligomeric state, and dispersity for every variant [4].
- Functional Assays: Perform targeted assays (e.g., viral neutralization assays for therapeutics, fluorescence measurements for reporters).
- Learn:
- Automated Data Analysis: Use open-source software to automatically analyze thousands of SEC chromatograms, standardizing the output [4].
- Data Integration: The quantitative, high-quality data from the "Test" phase is aggregated into a structured dataset.
- Model Refinement: This dataset is fed back into the AI design models to improve their predictive accuracy for the next DBTL cycle, creating a "self-driving" laboratory for protein engineering [4].
Historical Bottleneck: Before such integrated platforms, the "Learn" phase was stymied by a lack of standardized, high-quality experimental data produced at a scale that matches AI's design capabilities. The SAPP/DMX platform directly confronts this by generating the robust, large-scale data required to effectively train and refine AI models [4].
Diagram 1: The DBTL cycle in biological engineering.
Diagram 2: An integrated computational-experimental workflow to overcome the 'Learn' bottleneck.
The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential Research Reagents and Tools for Data-Driven Biology
| Reagent / Tool | Function | Application in Case Studies |
|---|---|---|
| Oligo Pools | Large, complex mixtures of synthetic DNA oligonucleotides used for cost-effective gene library construction. | DMX workflow uses them to build thousands of gene variants, reducing DNA synthesis costs by 5-8 fold [4]. |
| Golden Gate Assembly | A modular, efficient DNA assembly method that uses Type IIS restriction enzymes. | Used in the SAPP workflow with a ccdB "suicide gene" vector for high-fidelity (~90%), sequencing-free cloning [4]. |
| Auto-induction Media | Culture media formulated to automatically induce protein expression when cells reach a specific growth phase. | Used in SAPP's miniaturized parallel processing in 96-well plates to eliminate the need for manual induction, saving hands-on time [4]. |
| Size-Exclusion Chromatography (SEC) | A chromatography technique that separates molecules based on their size and shape. | In the SAPP platform, a parallelized SEC step provides simultaneous data on purity, yield, oligomeric state, and dispersity [4]. |
| Natural Language Processing (NLP) | A branch of AI that helps computers understand and interpret human language. | Used to analyze unstructured text from clinical notes, social media, and scientific literature for pharmacovigilance and DDI detection [16] [18]. |
| Spontaneous Reporting Systems (SRS) | Databases that collect voluntary reports of adverse drug events from healthcare professionals and patients. | Resources like FAERS and Vigibase are mined for signals of potential drug safety issues [18]. |
The "Learn" bottleneck has long been a formidable barrier in biological engineering, slowing the pace from discovery to application. Its roots lie in the multifaceted challenges of data integration, quality, and interpretation within the DBTL cycle. As the case studies in drug safety and protein engineering demonstrate, overcoming this bottleneck requires more than just advanced algorithms; it necessitates a holistic approach that includes the generation of standardized, high-quality experimental data at scale, the development of integrated computational-experimental platforms, and a critical, expert-driven interpretation of data-driven findings. The future of biological research hinges on continued innovation that tightens the DBTL loop, transforming the "Learn" phase from a historical impediment into a powerful engine for discovery.
From Code to Cell: Methodological Applications of DBTL in Strain and Therapy Development
High Throughput Screening (HTS) is a drug-discovery process widely used in the pharmaceutical industry that leverages specialized automation and robotics to quickly and economically assay the biological or biochemical activity of large collections of drug-like compounds [19]. This approach is particularly useful for discovering ligands for receptors, enzymes, ion-channels, or other pharmacological targets, and for pharmacologically profiling cellular or biochemical pathways of interest [19]. The core principle of HTS involves performing assays in "automation-friendly" microtiter plates with standardized formats such as 96, 384, or 1536 wells, enabling the rapid production of consistent, high-quality data while generating less waste due to smaller consumption of materials [19]. The integration of robotics and automation has transformed this field, allowing researchers to overcome previous limitations in manual handling techniques and significantly accelerating the pace of biological discovery and engineering.
Within the context of synthetic biology, HTS and laboratory robotics serve as critical enabling technologies for the Design-Build-Test-Learn (DBTL) cycle, a fundamental framework for systematically and iteratively developing and optimizing biological systems [1]. As synthetic biology has matured over the past two decades, the increased capacity for constructing biological systems has created unprecedented demands for testing capabilities that now exceed what manual techniques can deliver [20]. This surge has driven the establishment of biofoundries worldwide—specialized facilities where biological parts and systems can be built and tested rapidly through high-throughput automated assembly and screening methods [20]. These automated platforms leverage next-generation sequencing and mass spectrometry to collect large amounts of multi-omics data at the single-cell level, generating the extensive datasets necessary for advancing rational biological design [20].
The DBTL Cycle in Synthetic Biology
The Design-Build-Test-Learn (DBTL) cycle represents a systematic framework employed in synthetic biology for engineering biological systems to perform specific functions, such as producing biofuels, pharmaceuticals, or other valuable compounds [1]. This iterative development pipeline begins with the rational design of biological components, followed by physical construction of these designs, rigorous testing of their functionality, and finally learning from the results to inform the next design iteration [20]. A hallmark of synthetic biology is the application of rational principles to design and assemble biological components into engineered pathways, though the complex nature of biological systems often makes it difficult to predict the impact of introducing foreign DNA into a cell [1]. This uncertainty creates the need to test multiple permutations to obtain desired outcomes, a process dramatically accelerated through automation.
The past decade has seen remarkable advancements in the "design" and "build" stages of the DBTL cycle, driven largely by massive improvements in DNA sequencing and synthesis technologies that have significantly reduced both cost and turnaround time [20]. While sequencing a human genome cost approximately $10 million in 2007, the price has dropped to around $600 today, enabling researchers to sequence whole genomes of organisms and amass vast genomic databases that form the foundation for re-designing biological systems [20]. Concurrently, easing DNA synthesis costs and novel DNA assembly methodologies like Gibson assembly have overcome limitations of conventional cloning methods, enabling seamless assembly of combinatorial genetic parts and even entire synthetic chromosomes [20]. These developments, coupled with advances in genetic toolkits and genome editing techniques, have expanded the arsenal of organisms that can serve as chassis for synthetic biology applications.
The Learning Bottleneck and Machine Learning
Despite significant progress in the "build" and "test" phases of the DBTL cycle, synthetic biologists have faced substantial challenges in the "learn" stage due to the complexity, heterogeneity, and interconnected nature of biological systems [20]. While researchers can generate enormous amounts of biological data through automated high-throughput methods, extracting meaningful insights from these datasets has proven difficult. Many synthetic biologists still resort to top-down approaches based on likelihoods and trial-and-error to determine optimum designs, deviating from the field's aspiration to rationally design organisms from characterized genetic parts [20].
Machine learning (ML) has recently emerged as a promising approach to overcome the "learning" bottleneck in the DBTL cycle [20]. ML processes large datasets and generates predictive models by selecting appropriate features to represent phenomena of interest and uncovering previously unseen patterns among them. The technique has already demonstrated success in improving biological components like promoters and enzymes at the individual part level [20]. To advance synthetic biology further, ML must facilitate system-level prediction of biological designs with desired characteristics by elucidating associations between phenotypes and various combinations of genetic parts and genotypes. As explainable ML advances, it promises to provide both predictions and rationale for proposed designs, deepening our understanding of biological relationships and accelerating the learning stage of the DBTL cycle [20].
Automation Technologies for High-Throughput Implementation
Robotic Platforms and Workcells
Modern implementation of high-throughput screening relies on sophisticated robotic platforms configured into integrated workcells. The Wertheim UF Scripps Institute's robotics laboratory exemplifies this approach, occupying 1452 ft² and sharing two Kalypsys-GNF robotic platforms between HTS and Compound Management functions [19]. Similarly, Ginkgo Bioworks has developed Reconfigurable Automation Carts (RACs) that can be rearranged and configured to meet the specific needs of each experiment [21]. These systems incorporate a robotic arm and magnetic track that move sample plates from one piece of equipment to another, with the entire system controlled by integrated software [21]. Jason Kelly, CEO of Ginkgo Bioworks, describes their approach as creating automated robots capable of performing major molecular biology lab operations in a semi-standardized way, analogous to how unit operations revolutionized chemical engineering [21].
Commercial solutions like the HighRes Biosolutions ELEMENTS Screening Work Cell are built around mobile Nucleus FlexCarts that enable vertical integration of multiple screening workflow devices [22]. These systems feature pre-configured devices and editable templates or user-scripted protocols for high-throughput automation and optimized cell-based screening [22]. A key advantage of these modular systems is their flexibility—devices can be rapidly added or removed from the work cell as experimental needs evolve, and individual components can be quickly moved offline for manual use and maintenance without disturbing overall automated workflows [22]. This modularity extends to device compatibility, with systems designed to accommodate a range of preferred devices from various manufacturers while maintaining optimal functionality within automated workflows.
Essential Hardware Components
Fully automated screening workcells incorporate a carefully curated selection of specialized devices that work in concert to execute complex experimental protocols. The following table summarizes core components typically found in these systems:
Table 1: Essential Components of Automated Screening Workcells
| Component Category | Specific Examples | Function |
|---|---|---|
| Sample Storage & Retrieval | AmbiStore D Random Access Sample Storage Carousel | Delivers and stores labware in as few as 12 seconds to enhance efficiency and throughput [22] |
| Liquid Handling | Agilent Bravo, Tecan Fluent, Hamilton Vantage, or Beckman Echo Automated Liquid Handler | Precisely dispenses liquids across microplate formats [22] |
| Plate Management | Two LidValet High-Speed Delidding Hotels | Rapidly removes, holds, and replaces most microplate lids while the robotic arm tends to other tasks [22] |
| Detection & Analysis | Microplate Reader | Measures biological or biochemical activity in well plates [22] |
| Sample Processing | Microplate Washer, Automated Plate Sealer, Automated Plate Peeler | Performs essential plate processing steps [22] |
| Incubation & Storage | Automated Incubator | Maintains optimal growth conditions for cell-based assays [22] |
| Mixing & Preparation | Shakers, MicroSpin Automated Centrifuge | Prepares samples for analysis [22] |
These integrated components enable complete walk-away automation for complex screening protocols. For instance, systems can be configured with template protocols for specific applications like Cell Titer-Glo assays (completing 40 plates in approximately 8 hours), IP-1 Gq assays (completing 80 assay-ready plates in approximately 8 hours), or Transcreener protocols (completing 30 plates in approximately 8 hours) [22]. Users can modify these existing templates or design completely new protocols from scratch, choosing from a wide variety of 96-, 384-, and 1536-well plate options to meet their specific research requirements [22].
Experimental Protocols and Implementation
Knowledge-Driven DBTL for Dopamine Production
A recent implementation of the automated DBTL cycle demonstrates its power for strain optimization in synthetic biology. Researchers developing an Escherichia coli strain for dopamine production employed a "knowledge-driven DBTL" approach that combined upstream in vitro investigation with high-throughput ribosome binding site (RBS) engineering [11]. This methodology enabled both mechanistic understanding and efficient DBTL cycling, resulting in a dopamine production strain capable of producing dopamine at concentrations of 69.03 ± 1.2 mg/L (equivalent to 34.34 ± 0.59 mg/g biomass)—a 2.6 to 6.6-fold improvement over state-of-the-art in vivo dopamine production [11].
The experimental workflow began with in vitro cell lysate studies to assess enzyme expression levels in the potential dopamine production host before committing to full DBTL cycling [11]. This preliminary investigation provided crucial mechanistic insights that informed the subsequent design phase. Researchers then translated these in vitro findings to an in vivo environment through high-throughput RBS engineering, focusing particularly on how GC content in the Shine-Dalgarno sequence influences RBS strength [11]. The automated "build" phase involved DNA assembly and molecular cloning, while the "test" phase utilized cultivation experiments in minimal medium with precisely controlled components followed by analytical measurements to quantify dopamine production [11].
Automated Continuous Evolution Systems
The iAutoEvoLab platform represents another advanced implementation of laboratory automation for synthetic biology applications. This industrial-grade automation system serves as an all-in-one laboratory for programmable protein evolution in yeast, demonstrating high throughput, efficiency, and effectiveness through the evolution of diverse proteins including a DNA-binding protein (LmrA), a lactate sensor (LldR), and an RNA polymerase-capping enzyme fusion protein [23]. Such continuous evolution systems leverage completely automated workflows to perform multiple rounds of the DBTL cycle without manual intervention, dramatically accelerating the protein engineering process.
These automated evolution systems typically employ growth-coupled selection strategies where improved protein function directly correlates with enhanced cellular growth rates [23]. The platform autonomously manages the entire process from genetic diversification and transformation through cultivation, selection, and analysis. This approach enables the exploration of vast sequence spaces that would be impossible to screen manually, identifying beneficial mutations that enhance protein stability, activity, or specificity. The integration of automated analytics allows for real-time monitoring of evolutionary progress and intelligent decision-making about which lineages to pursue in subsequent evolution rounds.
Diagram 1: Automated DBTL cycle in synthetic biology, showing integration of laboratory robotics.
Research Reagent Solutions and Essential Materials
Successful implementation of automated high-throughput screening requires carefully selected research reagents and materials optimized for robotic compatibility. The following table details essential components used in automated synthetic biology workflows, particularly for strain engineering and compound production applications as demonstrated in the dopamine production case study [11]:
Table 2: Essential Research Reagents for Automated Strain Engineering
| Reagent Category | Specific Examples | Function in Workflow |
|---|---|---|
| Bacterial Strains | Escherichia coli DH5α (cloning strain), E. coli FUS4.T2 (production strain) | Provides biological chassis for genetic engineering and compound production [11] |
| Expression Plasmids | pET system (storage vector), pJNTN (crude cell lysate system and library construction) | Serves as vehicles for heterologous gene expression and pathway engineering [11] |
| Enzymes & Genetic Parts | 4-hydroxyphenylacetate 3-monooxygenase (HpaBC), l-DOPA decarboxylase (Ddc) | Catalyzes specific reactions in engineered metabolic pathways [11] |
| Media Components | 2xTY medium, SOC medium, Minimal medium with precise carbon sources | Supports cell growth and production under defined conditions [11] |
| Buffers & Solutions | Phosphate buffer (50 mM, pH 7), Reaction buffer for cell lysate systems | Maintains optimal pH and reaction conditions for enzymatic activity [11] |
| Antibiotics & Inducers | Ampicillin, Kanamycin, Isopropyl β-d-1-thiogalactopyranoside (IPTG) | Selects for transformed cells and controls gene expression induction [11] |
| Analytical Standards | Dopamine, l-tyrosine, l-DOPA | Enables quantification of metabolic products and pathway intermediates [11] |
These reagents must be formulated for compatibility with automated liquid handling systems, with particular attention to viscosity, stability, and concentration uniformity to ensure reproducible results across high-throughput experiments. Special consideration is given to reagents that will interface with sensitive detection systems such as microplate readers, where background fluorescence or absorbance can interfere with assay performance.
Data Management and Analysis in Automated Workflows
Control Software and Scheduling
Modern automated screening platforms rely on sophisticated software systems for seamless operation and management. Solutions like Cellario whole lab workflow automation software provide smooth workflow scheduling and walk-away convenience by ensuring all devices in the laboratory network are optimally configured and managed [22]. This software enables dynamic scheduling, implementation, and organization of devices and software across a lab or lab network while structuring sample utilization [22]. The interface allows users to design new protocols by modifying existing template protocols or creating completely new ones from scratch, with support for a wide variety of labware definitions and plate formats [22].
These software platforms must balance flexibility with reproducibility, allowing researchers to adapt protocols to specific needs while maintaining rigorous standards for experimental consistency. This is particularly important in regulated environments like pharmaceutical development, where documentation and protocol adherence are critical. The software typically includes features for real-time monitoring of instrument status, error logging and recovery procedures, and data tracking throughout the entire experimental workflow. Integration with laboratory information management systems (LIMS) enables comprehensive sample tracking from initial preparation through final data analysis.
Data Analysis and Machine Learning Integration
The massive datasets generated by automated HTS platforms create both opportunities and challenges for data analysis. As Kelly of Ginkgo Bioworks notes, automated robotics enable better development of and integration with artificial intelligence models and neural networks [21]. This synergy between automation and machine learning is particularly powerful for distilling complex biological information and establishing core design principles for rational engineering of organisms [20].
Machine learning approaches excel at identifying patterns within high-dimensional datasets that might escape conventional statistical analysis. In synthetic biology applications, ML has been successfully used to improve biological components such as promoters and enzymes at the genetic part level, where sufficient dataset sizes are available [20]. The next frontier involves facilitating system-level prediction of biological designs with desired characteristics by elucidating associations between phenotypes and various combinations of genetic parts and genotypes. Advances in explainable ML are particularly valuable as they provide both predictions and rationale for proposed designs, accelerating the learning stage of the DBTL cycle while simultaneously deepening fundamental understanding of biological systems [20].
Diagram 2: Data flow from HTS robotics to machine learning-enabled biological design.
The integration of high-throughput screening and laboratory robotics within the DBTL framework has fundamentally transformed synthetic biology and drug discovery workflows. These automated systems have dramatically increased throughput while reducing human error and variability, enabling researchers to tackle biological engineering challenges at unprecedented scales [19] [21]. The continued advancement of these technologies, particularly through tighter integration with machine learning and artificial intelligence, promises to further accelerate the pace of biological discovery and engineering.
Looking forward, we can anticipate several key developments in automated biological engineering. First, the continued refinement of biofoundries and global collaborations through organizations like the Global Biofoundry Alliance will establish common standards for designing and generating ML-friendly data [20]. Second, advances in explainable machine learning will increasingly bridge the gap between data-driven predictions and mechanistic understanding, enabling true rational design of biological systems [20]. Finally, the application of these automated platforms to increasingly complex biological challenges—from engineered microbes for sustainable chemical production to diagnostic and therapeutic microbes that can identify diseases in situ and produce drugs in vivo—will expand the impact of synthetic biology on addressing crucial societal problems [20].
The fusion of laboratory automation, high-throughput screening, and machine learning within the DBTL cycle represents more than just a technical improvement—it constitutes a fundamental shift in how we approach biological engineering. By making biology easier to engineer [21], these integrated platforms promise to unlock new capabilities in medicine, manufacturing, agriculture, and environmental sustainability. As these technologies continue to mature and become more accessible, they will empower researchers to tackle biological challenges with unprecedented precision, efficiency, and scale, ultimately fulfilling synthetic biology's promise as a truly engineering discipline for biological systems.
The field of biological engineering is increasingly turning to microbial cell factories as a sustainable and programmable platform for the production of valuable metabolites. This paradigm shift is particularly evident in the biosynthesis of complex organic compounds like dopamine and biopolymers such as polyhydroxyalkanoates (PHA), which have significant applications in medicine and materials science. The design-build-test-learn (DBTL) cycle has emerged as a foundational framework in synthetic biology, enabling the systematic engineering of biological systems through iterative refinement. This case study examines the application of the DBTL cycle in engineering microbial platforms for the production of dopamine and PHA, highlighting the integrated methodologies that bridge computational design, genetic construction, phenotypic characterization, and data-driven learning.
The DBTL cycle represents a structured approach to biological engineering that transforms traditional linear research and development into an iterative, learning-driven process. In the context of metabolite production, this framework allows researchers to navigate the complexity of cellular metabolism by systematically addressing bottlenecks in biosynthetic pathways. As demonstrated in recent advances in Escherichia coli and Cupriavidus necator engineering, the implementation of automated DBTL workflows has dramatically accelerated strain development, leading to significant improvements in production titers, rates, and yields (TRY) [24] [25]. This case study will provide an in-depth technical analysis of pathway design, host engineering, and process optimization for dopamine and PHA production, serving as a model for the application of DBTL cycles in biological engineering research.
The DBTL Cycle in Biological Engineering
Conceptual Framework and Workflow Integration
The DBTL cycle operates as an integrated framework that connects computational design with experimental implementation through four interconnected phases: (1) Design phase employs computational tools to model metabolic pathways and predict genetic constructs; (2) Build phase implements automated genetic engineering to construct designed variants; (3) Test phase characterizes constructed strains through high-throughput screening and multi-omics analysis; and (4) Learn phase applies statistical modeling and machine learning to extract insights for subsequent design iterations [26]. This cyclical process has become increasingly automated through biofoundries—integrated facilities that combine laboratory automation, robotics, and data science to accelerate biological design.
Recent implementations demonstrate the power of fully automated DBTL cycles. Carbonell et al. achieved a 500-fold increase in (2S)-pinocembrin production through just two DBTL cycles, screening only 65 variants rather than thousands through targeted design [26]. Similarly, autonomous DBTL platforms like BioAutomata and AutoBioTech have successfully improved lycopene production by 1.77-fold through machine learning-guided iterations [26]. These successes highlight how the DBTL framework efficiently navigates the vast combinatorial space of biological engineering problems.
Knowledge-Driven DBTL Implementation
A significant advancement in DBTL methodology is the "knowledge-driven" approach that incorporates upstream in vitro investigations before embarking on full DBTL cycles [24] [25]. This strategy uses cell-free transcription-translation systems and crude cell lysates to rapidly prototype enzyme combinations and relative expression levels without the constraints of cellular membranes and internal regulation. The mechanistic insights gained from these preliminary experiments inform the initial design phase, reducing the number of iterations needed to achieve optimal performance.
The implementation of knowledge-driven DBTL cycles has demonstrated remarkable efficiency improvements. In one case, researchers developed a highly efficient dopamine production strain by combining in vitro pathway optimization with high-throughput ribosome binding site (RBS) engineering, achieving a 2.6 to 6.6-fold improvement over previous in vivo production systems [25]. This approach successfully translated insights from cell-free systems to living cells, demonstrating the value of incorporating mechanistic understanding into the DBTL framework.
Microbial Production of Dopamine
Biosynthetic Pathways and Enzyme Engineering
Dopamine (3,4-dihydroxyphenethylamine) is a catecholamine neurotransmitter with applications in emergency medicine, cancer diagnosis and treatment, lithium anode production, and wastewater treatment [24] [25]. In natural systems, dopamine biosynthesis occurs in catecholaminergic neurons through a two-step pathway beginning with the hydroxylation of L-tyrosine to L-DOPA by tyrosine hydroxylase (TH), followed by decarboxylation to dopamine by aromatic L-amino acid decarboxylase (AADC) [27] [28]. Both enzymes are highly regulated, with TH serving as the rate-limiting step under physiological conditions [29].
For microbial production, researchers have engineered heterologous pathways in E. coli using a slightly different approach. The native E. coli gene encoding 4-hydroxyphenylacetate 3-monooxygenase (HpaBC) converts L-tyrosine to L-DOPA, which is subsequently decarboxylated to dopamine by L-DOPA decarboxylase (Ddc) from Pseudomonas putida [24]. This pathway bypasses the need for TH, which requires the cofactor tetrahydrobiopterin (BH4) that is not naturally produced in E. coli. The enzymatic mechanism of AADC involves pyridoxal phosphate (PLP) as a cofactor, forming a Schiff base with the substrate during decarboxylation [28]. Optimal decarboxylation conditions vary between substrates, with DOPA decarboxylation optimized at pH 6.7 and 0.125 mM PLP, while 5-HTP decarboxylation prefers pH 8.3 and 0.3 mM PLP [28].
Figure 1: Microbial Dopamine Biosynthesis Pathway. The pathway illustrates the two-step conversion of L-tyrosine to dopamine using heterologous enzymes HpaBC and Ddc in E. coli.
Host Engineering and Pathway Optimization
Successful dopamine production requires extensive host engineering to ensure adequate precursor supply. E. coli FUS4.T2 has been engineered as a production host with enhanced L-tyrosine availability through deletion of the transcriptional dual regulator TyrR and mutation of the feedback inhibition in chorismate mutase/prephenate dehydrogenase (TyrA) [24]. These modifications increase carbon flux through the shikimate pathway toward L-tyrosine, the direct precursor for dopamine synthesis.
A knowledge-driven DBTL approach was implemented to optimize dopamine production, beginning with in vitro testing in crude cell lysates to determine optimal relative expression levels of HpaBC and Ddc [25]. These insights were then translated to the in vivo environment through high-throughput RBS engineering to fine-tune expression levels. By modulating the Shine-Dalgarno sequence without interfering with secondary structures, researchers developed a dopamine production strain capable of producing 69.03 ± 1.2 mg/L dopamine, equivalent to 34.34 ± 0.59 mg/g biomass [24] [25]. This represents a significant improvement over previous in vivo production systems, demonstrating the power of targeted pathway optimization.
Experimental Protocol: Dopamine Production and Analysis
Strain Construction:
- Start with engineered E. coli FUS4.T2 with enhanced L-tyrosine production (ΔtyrR, tyrA-fbr) [24].
- Amplify hpaBC (from E. coli) and ddc (from P. putida) genes with appropriate RBS sequences using primers with overhangs for assembly.
- Assemble genes into a suitable expression vector (e.g., pET-based) with inducible promoter (lac or T7) using Gibson Assembly or Golden Gate cloning.
- Transform constructed plasmid into production host using electroporation and plate on selective media (LB + appropriate antibiotic).
- Verify constructs by colony PCR and Sanger sequencing.
Cultivation and Production:
- Inoculate single colonies into 5 mL LB medium with antibiotic and grow overnight at 37°C, 250 rpm.
- Dilute overnight culture 1:100 into minimal medium containing: 20 g/L glucose, 10% 2xTY medium, 2.0 g/L NaH2PO4·2H2O, 5.2 g/L K2HPO4, 4.56 g/L (NH4)2SO4, 15 g/L MOPS, 50 μM vitamin B6, 5 mM phenylalanine, 0.2 mM FeCl2, and 0.4% (v/v) trace elements [24].
- Grow cultures at 37°C, 250 rpm until OD600 reaches 0.6-0.8.
- Induce expression with 1 mM IPTG and reduce temperature to 30°C.
- Continue incubation for 24-48 hours with monitoring of cell density and metabolite production.
Analytical Methods:
- Biomass measurement: Monitor OD600 at regular intervals. For cell dry weight, pellet known culture volume, wash with distilled water, and dry at 80°C to constant weight.
- Dopamine quantification: Centrifuge culture samples at 13,000 × g for 5 min, filter supernatant through 0.22 μm membrane, and analyze by HPLC with C18 column and electrochemical or UV detection.
- Metabolite profiling: Use LC-MS for comprehensive analysis of pathway intermediates (L-tyrosine, L-DOPA) and byproducts.
Table 1: Key Research Reagents for Microbial Dopamine Production
| Reagent/Component | Function/Application | Example Sources |
|---|---|---|
| E. coli FUS4.T2 | Production host with enhanced L-tyrosine synthesis | [24] |
| HpaBC enzyme | Converts L-tyrosine to L-DOPA | Native E. coli gene [24] |
| Ddc enzyme | Decarboxylates L-DOPA to dopamine | Pseudomonas putida [24] |
| Minimal medium with MOPS buffer | Defined cultivation conditions | [24] |
| Vitamin B6 (pyridoxal phosphate precursor) | Cofactor for Ddc enzyme | [24] [28] |
| FeCl2 | Cofactor for HpaBC enzyme | [24] |
Microbial Production of Polyhydroxyalkanoates (PHA)
PHA Diversity and Biosynthetic Pathways
Polyhydroxyalkanoates are a family of biodegradable polyesters synthesized by numerous microorganisms as intracellular carbon and energy storage compounds. Over 300 bacterial species, including Cupriavidus necator, Pseudomonas putida, and various halophiles, naturally accumulate PHA under nutrient limitation with excess carbon [30]. These biopolymers are classified based on the carbon chain length of their monomeric units: short-chain-length (scl) PHA (C3-C5 monomers) including poly(3-hydroxybutyrate) (PHB), and medium-chain-length (mcl) PHA (C6-C14 monomers) with elastomeric properties [30].
The biosynthesis of PHA involves three key enzymes: (1) 3-ketothiolase (PhaA) catalyzes the condensation of two acetyl-CoA molecules to acetoacetyl-CoA; (2) acetoacetyl-CoA reductase (PhaB) reduces acetoacetyl-CoA to (R)-3-hydroxybutyryl-CoA; and (3) PHA synthase (PhaC) polymerizes the hydroxyacyl-CoA monomers into PHA [30]. In C. necator, this pathway produces the homopolymer PHB, while P. putida employs different PHA synthases that incorporate longer-chain monomers from fatty acid β-oxidation or de novo synthesis [30].
Host Selection and Engineering Strategies
Different microbial hosts offer distinct advantages for PHA production. Cupriavidus necator H16 is renowned for high PHB accumulation, reaching up to 71% of cell dry weight on fructose in shake flasks and over 90% on waste rapeseed oil in bioreactors [30]. This organism primarily employs the Entner-Doudoroff and butanoate pathways for sugar metabolism [30]. In contrast, Pseudomonas putida KT2440 produces mcl-PHAs with lower crystallinity and melting temperatures but superior elastomeric properties, with elongation at break reaching 300-500% [30].
Recent engineering efforts have expanded the range of hosts and PHA types produced. The极端微生物Halomonas (salt-loving bacteria) isolated from the extreme environment of China's Lake Ading has been engineered for industrial PHA production [31]. This organism grows under alkaline and high-salt conditions that minimize microbial contamination, enabling continuous bioprocessing. Through the development of customized genetic tools, researchers have created Halomonas strains that accumulate PHA up to 80% of cell dry weight and can be cultivated in unsterile conditions [31].
Yeast platforms have also been engineered for PHA production. Yarrowia lipolytica has been modified to synthesize poly(3-hydroxybutyrate-co-4-hydroxybutyrate) [P(3HB-co-4HB)] through compartmentalized metabolic engineering [32]. By localizing 4HB synthesis to mitochondria and PHB synthesis to the cytosol, researchers achieved copolymer production with 4HB monomer ratios adjustable from 9.17 to 45.26 mol% by modifying media composition [32]. In 5-L bioreactors, the engineered strain produced 18.61 g/L P(3HB-co-4HB) at 19.18% cellular content [32].
Table 2: Comparison of Microbial Platforms for PHA Production
| Parameter | Cupriavidus necator H16 | Pseudomonas putida KT2440 | Engineered Halomonas | Yarrowia lipolytica |
|---|---|---|---|---|
| PHA Type | scl-PHA (PHB) | mcl-PHA | scl-PHA & copolymers | scl-PHA & copolymers |
| Max PHA Content | 71-90% CDW | 18-22% CDW | ~80% CDW | 19.18% CDW |
| Carbon Sources | Fructose, plant oils | Glucose, fructose | Various waste streams | Glucose, glycerol |
| Cultivation Requirements | Standard conditions | Standard conditions | High salt, alkaline pH | Standard conditions |
| Key Features | High productivity, established industrial use | Elastic polymer properties | Contamination-resistant, open fermentation | Eukaryotic host, compartmentalization |
| Polymer Properties | High crystallinity (30-90%), Tm = 175-180°C | Low crystallinity (20-40%), Tm = 30-80°C | Tunable properties | Tunable copolymer composition |
| Challenges | Sterile cultivation required | Lower productivity | Specialized bioreactor materials | Lower productivity than bacterial systems |
Experimental Protocol: PHA Production and Characterization
Microbial Cultivation for PHA Production:
- Strain preparation: Maintain production strains (C. necator H16, P. putida KT2440, or engineered variants) on nutrient agar plates with appropriate antibiotics.
- Pre-culture preparation: Inoculate single colonies into 5-10 mL rich medium (e.g., Nutrient Broth or Lysogeny Broth) and incubate overnight at 30°C, 250 rpm.
- Production cultivation: Dilute pre-culture into minimal salt medium (MSM) with carbon source (e.g., 20 g/L glucose or fructose) and appropriate C:N:P ratio to trigger PHA accumulation.
- Bioreactor conditions: For scale-up, use controlled bioreactors with dissolved oxygen >20%, pH 7.0, temperature 30°C. For Halomonas strains, maintain pH ~9.0 and add NaCl to 3-5% [31].
- Harvesting: Centrifuge cultures at late stationary phase (typically 48-72 hours), wash cells with distilled water, and freeze-dry for PHA extraction.
PHA Extraction and Analysis:
- Solvent extraction: Mix lyophilized biomass with chloroform (1:10 w/v) and reflux at 60-70°C for 2-4 hours. Filter to remove cell debris, then concentrate by rotary evaporation.
- Precipitation: Add concentrated PHA solution to 10 volumes of cold methanol or ethanol with stirring. Collect precipitated PHA by filtration and dry under vacuum.
- Monomer composition: Analyze by GC-MS after methanolysis of polymer samples (treat with acidic methanol at 100°C for 4 hours).
- Molecular weight: Determine by gel permeation chromatography (GPC) using polystyrene standards and chloroform as mobile phase.
- Thermal properties: Analyze by differential scanning calorimetry (DSC) with heating rate of 10°C/min under nitrogen atmosphere.
Film Preparation and Characterization:
- Solution casting: Dissolve purified PHA in chloroform (2-5% w/v), filter through 0.45 μm PTFE membrane, and cast onto glass plates.
- Film formation: Allow solvent evaporation under controlled conditions (25°C, 50% RH) for 24 hours, then vacuum-dry at 40°C to constant weight.
- Characterization: Analyze mechanical properties (tensile testing), thermal behavior (DSC, TGA), and crystallinity (XRD, FTIR).
Figure 2: PHA Biosynthetic Pathways in Microbial Hosts. The diagram shows the convergence of short-chain-length (PHB) and medium-chain-length PHA biosynthesis from central metabolic precursors.
Table 3: Research Reagents for Microbial PHA Production
| Reagent/Component | Function/Application | Example Sources/Strains |
|---|---|---|
| Cupriavidus necator H16 | High-PHB accumulating strain | DSM 428, ATCC 17699 [30] |
| Pseudomonas putida KT2440 | mcl-PHA producing strain | DSM 6125, ATCC 47054 [30] |
| Engineered Halomonas | Contamination-resistant PHA producer | Tsinghua University [31] |
| Minimal Salt Medium (MSM) | Defined medium for PHA accumulation | [30] |
| Chloroform | Solvent for PHA extraction and purification | [30] |
| PHA synthase (PhaC) | Key polymerase enzyme | C. necator, P. aeruginosa [30] [32] |
Integrated DBTL Workflows for Strain Optimization
Case Study: DBTL Implementation for Dopamine Production
The development of high-performance dopamine production strains exemplifies the power of integrated DBTL workflows. Researchers implemented a knowledge-driven DBTL cycle that began with in vitro prototyping in crude cell lysates to determine optimal expression levels for HpaBC and Ddc enzymes [24] [25]. This preliminary investigation provided mechanistic insights that informed the initial design phase, significantly reducing the number of design iterations required.
In the build phase, high-throughput RBS engineering was employed to create a library of variants with precisely tuned expression levels. Rather than random screening, the design focused on modulating the GC content in the Shine-Dalgarno sequence while maintaining secondary structure stability [24]. The test phase involved automated cultivation in 96-well format with HPLC analysis of dopamine production, generating quantitative data for 69.03 ± 1.2 mg/L dopamine production [25]. In the learn phase, the correlation between SD sequence features and protein expression levels was modeled to inform the next design iteration, ultimately achieving a 6.6-fold improvement in specific productivity compared to previous reports [25].
Case Study: Industrial PHA Production Scaling
The scale-up of PHA production from laboratory curiosity to industrial reality demonstrates DBTL principles applied across multiple scales. Professor Guo-Qiang Chen's team at Tsinghua University spent three decades developing a competitive PHA production platform using engineered Halomonas [31]. Their approach involved multiple DBTL cycles addressing different challenges: (1) initial discovery and genetic tool development for the non-model organism; (2) metabolic engineering to enhance PHA yield and content; (3) process engineering to enable open, unsterile fermentation; and (4) integration with downstream processing for efficient polymer recovery.
This extended DBTL implementation has led to the establishment of a 10,000-ton PHA production line in Hubei, China, with plans to expand to 30,000-ton capacity [31]. The success of this platform relied on addressing both biological and engineering constraints through iterative learning, including the development of specialized bioreactors to address oxygen transfer limitations and morphological engineering to facilitate cell separation [31]. The project exemplifies how DBTL cycles can bridge fundamental research to industrial implementation, reducing production costs from ¥50,000 to ¥30,000 per ton [31].
Figure 3: Knowledge-Driven DBTL Cycle with In Vitro Prototyping. The workflow illustrates how preliminary in vitro investigations inform the initial design phase of the DBTL cycle.
The engineering of microbial cell factories for dopamine and PHA production illustrates the transformative potential of systematic DBTL approaches in biological engineering. Future advances will likely come from several converging technological developments: (1) the increasing integration of artificial intelligence and machine learning throughout the DBTL cycle, particularly in the design and learn phases; (2) the expansion of biofoundry capabilities with greater automation and parallel processing; (3) the development of more sophisticated models that incorporate multi-omics data and kinetic parameters; and (4) the application of these approaches to non-model organisms with native abilities to utilize low-cost feedstocks.
For dopamine production, future research directions include the engineering of complete de novo pathways from simple carbon sources, dynamic regulation to balance precursor flux, and the extension to dopamine-derived compounds such as norepinephrine and epinephrine. In the PHA field, the focus is on expanding the range of monomer compositions, reducing production costs through waste valorization, and engineering polymer properties for specific applications. The continued refinement of DBTL methodologies will accelerate progress in both areas, potentially enabling the sustainable production of an expanding range of valuable metabolites and materials.
In conclusion, this case study demonstrates that the DBTL cycle provides an essential framework for tackling the complexity of biological systems engineering. Through iterative design, construction, testing, and learning, researchers can systematically overcome the limitations of natural metabolic pathways and cellular regulation. The examples of dopamine and PHA production highlight how this approach leads to tangible improvements in production metrics while generating fundamental biological insights that inform future engineering efforts. As DBTL methodologies become more sophisticated and widely adopted, they will undoubtedly drive further innovations in microbial cell factory development for diverse applications in medicine, materials, and industrial biotechnology.
Chimeric Antigen Receptor (CAR)-T cell therapy represents a transformative breakthrough in cancer immunotherapy, yet its effectiveness, particularly against solid tumors, remains limited by challenges such as tumor heterogeneity and suboptimal CAR designs. This case study explores a pioneering, AI-informed approach developed by researchers at St. Jude Children's Research Hospital for designing superior tandem CARs. Framed within the evolving paradigm of the Design-Build-Test-Learn (DBTL) cycle in biological engineering, the research demonstrates how shifting the "Learn" phase to the forefront—creating an LDBT cycle—can dramatically accelerate the development of effective immunotherapies. By leveraging a computational pipeline to screen thousands of theoretical CAR constructs in silico, the team successfully generated and validated a tandem CAR that completely cleared heterogeneous tumors in preclinical models, outperforming conventional single-target CARs. This work underscores the critical role of artificial intelligence and machine learning in overcoming the bottlenecks of traditional DBTL cycles, paving the way for more precise and powerful cell therapies.
The DBTL Framework in Synthetic Biology
Synthetic biology is defined by the iterative Design-Build-Test-Learn (DBTL) cycle, a systematic framework for engineering biological systems [20] [1]. This workflow begins with the Design of genetic parts or systems based on domain knowledge and computational modeling. In the Build phase, DNA constructs are synthesized and assembled into vectors before being introduced into a cellular or cell-free characterization system. The constructed biological systems are then experimentally evaluated in the Test phase to measure performance against the design objectives. Finally, data from testing is analyzed in the Learn phase to inform the next round of design, creating a continuous loop of refinement [2] [20]. While this approach has driven significant advancements, the field has historically faced a bottleneck in the "Learn" stage due to the complexity of biological systems and the challenge of extracting actionable design principles from large, heterogeneous datasets [20].
The Challenge of CAR-T Cell Therapy
CAR-T cell therapy harnesses the patient's own immune cells, engineering them to express synthetic receptors that target cancer-specific proteins. Despite remarkable success in treating hematological malignancies, CAR-T cells have been less effective against solid and brain tumors [33] [34]. A primary reason is tumor heterogeneity—the fact that cancer cells do not uniformly express the same surface proteins. CAR-T cells targeting a single antigen can miss malignant cells that do not express that protein, leading to tumor escape and relapse [33]. To address this, researchers have developed bi-specific tandem CARs capable of targeting two cancer-related antigens simultaneously. However, optimizing the design of these complex receptors has proven to be a "time-consuming, labor-intensive, and expensive challenge," often resulting in constructs with poor surface expression and suboptimal cancer-killing ability [33].
The AI-Informed Shift: From DBTL to LDBT
Recent advances in machine learning (ML) are catalyzing a paradigm shift in engineering biology. Rather than treating "Learn" as the final step that depends on data generated from a full Build-Test cycle, researchers are now proposing an LDBT cycle, where "Learning" precedes "Design" [2]. This approach leverages powerful ML models trained on vast biological datasets—such as protein sequences, structures, and evolutionary relationships—to make informed, zero-shot predictions about functional designs before any wet-lab experimentation begins [2].
This LDBT model was central to the St. Jude study. The team employed an AI-informed computational pipeline that could screen approximately 1,000 theoretical tandem CAR designs in a matter of days, a process that would take many years using traditional lab-based methods [33]. The algorithm was trained on the structural and biophysical features of known effective CARs, including properties like protein folding stability and aggregation tendency. It synthesized these features into a single "fitness" score predicting CAR expression and functionality, allowing researchers to rank and select the most promising candidates for experimental validation [33]. This exemplifies the LDBT paradigm: first Learning from existing data and models, then Designing optimal constructs in silico, before moving to the Build and Test phases.
Case Study: Computational Design of a Tandem CAR Targeting Pediatric Brain Tumors
Experimental Design and Workflow
The research team at St. Jude sought to design a superior tandem CAR targeting two proteins associated with pediatric brain tumors: B7-H3 and IL-13Rα2 [33]. Their experimental workflow is a prime example of the integrated LDBT cycle.
Learn & Design Phase: Computational Screening and Optimization
- Objective: Identify a tandem CAR construct with high surface expression and strong anti-tumor function.
- Computational Method: The team developed a pipeline that screened numerous theoretical tandem CAR designs. The AI algorithm was informed by structural and biophysical features of known functional CARs [33].
- Fitness Scoring: The model calculated a composite "fitness" score based on factors including:
- Predicted protein folding stability
- Tendency to aggregate
- Other structural and functional features
- Output: The top-ranked tandem CAR candidates were selected for further experimental validation.
Table 1: Key Features of the AI Screening Pipeline
| Feature | Description | Impact |
|---|---|---|
| Screening Scale | ~1,000 constructs screened in silico [33] | Dramatically accelerates design phase |
| Processing Time | Completed in days [33] | Versus years for traditional lab-based methods |
| Fitness Criteria | Protein stability, aggregation propensity, structural features [33] | Predicts expression and functionality |
| Output | Ranked list of top CAR candidates [33] | Guides focused experimental efforts |
Build Phase: Molecular Construction
- Vector System: The optimized CAR sequences were cloned into appropriate viral vectors for T-cell transduction [33].
- Cell Culture: Human T cells were isolated and activated ex vivo.
- Transduction: T cells were genetically engineered to express the top-ranked tandem CAR constructs via viral transduction, generating the final CAR-T cell product for testing [33].
Test Phase: Preclinical Validation
The functionality of the computationally optimized CARs was rigorously tested through a series of experiments:
- Surface Expression Validation: Confirmation that the optimized CAR was properly expressed on the T cell surface, overcoming a key failure mode of previous designs [33].
- In Vitro Cytotoxicity: Assessment of the CAR-T cells' ability to kill cancer cells expressing one or both target antigens [33].
- In Vivo Efficacy: Evaluation in mouse models bearing heterogeneous tumors composed of a mix of cells expressing both targets, one target, or neither target, thereby mimicking clinical tumor heterogeneity [33].
Diagram 1: The AI-driven LDBT workflow for CAR design. The process begins with Learning from diverse datasets to inform the AI model, which then powers the in-silico Design of CARs. The best designs are Built and Tested in a focused preclinical validation stage.
Key Findings and Quantitative Results
The preclinical validation yielded compelling evidence for the success of the AI-optimized tandem CAR.
- Tumor Eradication: The most compelling result was that the computationally optimized tandem CAR completely cleared tumors in four out of five mice (80%) [33].
- Control Group Failure: In stark contrast, "all heterogeneous tumors treated with single-targeted CAR T cells grew back" [33].
- Superior Functionality: The study further demonstrated that the optimized CARs "killed cancer cells better than the non-optimized tandem CARs" across multiple designs, confirming the generalizability of the approach [33].
Table 2: Preclinical Efficacy Results of AI-Optimized Tandem CAR
| Treatment Group | Tumor Clearance Rate | Functional Performance |
|---|---|---|
| AI-optimized Tandem CAR | 80% (4 out of 5 mice) [33] | Superior cancer cell killing |
| Single-Target CARs | 0% (All tumors regrew) [33] | Failed to control heterogeneous tumors |
| Non-optimized Tandem CAR | Not Specified (Poor) | Suboptimal cancer cell killing [33] |
The Scientist's Toolkit: Essential Research Reagents and Materials
The successful implementation of this AI-driven CAR design pipeline relied on a suite of critical research reagents and platforms.
Table 3: Key Research Reagent Solutions for AI-Driven CAR-T Cell Development
| Reagent / Platform | Function in the Workflow |
|---|---|
| AI/ML Protein Design Tools | In silico screening and optimization of protein sequences for stability and function. Tools like Rosetta were used in related studies for designing immune-modulating proteins [35]. |
| Cell-Free Expression Systems | Rapid, high-throughput synthesis and testing of protein variants without the constraints of cellular systems, enabling megascale data generation for model training [2]. |
| Viral Vector Systems | Delivery and stable genomic integration of the engineered CAR construct into human T cells [33] [34]. |
| Liquid Handling Robots & Microfluidics | Automation of the Build and Test phases, allowing for high-throughput assembly and screening, which is essential for generating robust datasets [2]. |
| Next-Generation Sequencing | Verification of synthesized DNA constructs and tracking of CAR-T cell populations [1]. |
Detailed Experimental Protocol
This section outlines the core methodologies cited in the featured case study, providing a replicable framework for AI-driven CAR development.
Protocol 1: Computational Screening of Tandem CAR Libraries
- Model Training: Train a machine learning algorithm on a curated dataset of known functional CARs. Input features should include predicted structural and biophysical properties such as folding stability, aggregation propensity, and solvent accessibility [33] [2].
- Library Generation: Computationally generate a large library (e.g., ~1,000 variants) of theoretical tandem CAR sequences by varying key structural domains and linkers [33].
- Fitness Scoring: Use the trained model to analyze each variant in the library and output a composite fitness score predicting cell surface expression and functional potency [33].
- Candidate Selection: Rank all variants by their fitness score and select the top-performing candidates (e.g., top 5-10) for experimental synthesis and validation [33].
Protocol 2: In Vivo Validation Against Heterogeneous Tumors
- Tumor Model Generation: Establish a xenograft mouse model by implanting cancer cells. To model clinical heterogeneity, use a mixture of tumor cells that express: a) both target antigens (B7-H3 and IL-13Rα2), b) only antigen A, c) only antigen B, and d) neither antigen [33].
- CAR-T Cell Administration: Once tumors are established, randomly allocate mice to treatment groups. Administer a single dose of the experimental AI-optimized tandem CAR-T cells, control single-target CAR-T cells, or a non-targeted T-cell control via tail vein injection [33].
- Tumor Monitoring: Monitor tumor volume regularly using caliper measurements or in vivo imaging (e.g., bioluminescence) over a period of several weeks [33].
- Endpoint Analysis: Assess primary endpoints such as tumor growth kinetics and the rate of complete tumor regression. Analyze T cell persistence and functionality in blood and tumor tissues at the end of the study [33].
This case study demonstrates a successful application of an AI-informed LDBT cycle to overcome a significant hurdle in cancer immunotherapy: the design of effective multi-targeted CARs. By placing Learning at the beginning of the cycle, researchers at St. Jude were able to rapidly navigate a vast design space in silico and identify tandem CAR constructs with a high probability of success. The resulting AI-optimized CAR demonstrated superior functionality and achieved complete tumor clearance in most animals, a result that single-target CARs could not match. This work validates the LDBT paradigm as a powerful framework for accelerating the development of complex biological therapeutics. It highlights that the future of synthetic biology and immunotherapy lies in the deep integration of computational and experimental science, bringing us closer to the day when challenging solid tumors can be consistently and effectively treated with cell therapy.
Diagram 2: Structure of an optimized bi-specific tandem CAR. The receptor contains two single-chain variable fragments (scFvs) for targeting different tumor antigens (B7-H3 and IL-13Rα2), connected via hinge and transmembrane domains to intracellular co-stimulatory and activation signaling domains.
Integrating Multi-Omics Data for Holistic System Design
The integration of multi-omics data represents a paradigm shift in biological engineering, enabling a more comprehensive understanding of complex biological systems. This technical guide details the methodologies and computational strategies for effectively merging disparate omics datasets—including genomics, transcriptomics, proteomics, and epigenomics—within the framework of the Design-Build-Test-Learn (DBTL) cycle. By leveraging advanced machine learning algorithms and high-throughput analytical platforms, researchers can now navigate the complexity of biological systems with unprecedented resolution. This whitepaper provides a structured approach to multi-omics integration, featuring quantitative comparison tables, detailed experimental protocols, specialized visualization frameworks, and essential research reagent solutions to equip scientists with the tools necessary for holistic system design in therapeutic development and basic research.
The Design-Build-Test-Learn (DBTL) cycle provides a systematic framework for engineering biological systems, with recent advances proposing a shift to "LDBT" where Learning precedes Design through machine learning [2]. Multi-omics integration strengthens this framework by providing comprehensive data layers that inform each stage of the cycle. During the Design phase, integrated multi-omics data reveals novel cell subtypes, cell interactions, and interactions between different omic layers leading to gene regulatory and phenotypic outcomes [36]. This enables more informed selection of biological parts and system architecture. The Build phase leverages high-throughput technologies to generate biological constructs, while the Test phase utilizes multi-omics readouts for comprehensive system characterization. Finally, the Learn phase employs sophisticated computational tools to extract meaningful patterns from integrated datasets, informing the next design iteration [36].
The fundamental challenge in multi-omics integration stems from the inherent differences in data structure, scale, and noise characteristics across modalities. For instance, scRNA-seq can profile thousands of genes, while current proteomic methods typically measure only about 100 proteins, creating feature imbalance [36]. Furthermore, biological correlations between modalities don't always follow conventional expectations—actively transcribed genes should have greater open chromatin accessibility, but the most abundant protein may not correlate with high gene expression [36]. These disconnects make integration technically challenging and necessitate specialized computational approaches tailored to specific data types and research questions.
Computational Strategies for Multi-Omics Integration
Integration Typology and Tool Classification
Multi-omics integration strategies can be categorized based on the nature of the input data, which determines the appropriate computational approach [36]:
- Matched (Vertical) Integration: Merges data from different omics within the same set of samples or single cells, using the cell itself as the anchor. This approach is ideal for technologies that concurrently profile multiple modalities from the same cell.
- Unmatched (Diagonal) Integration: Combines omics data drawn from distinct cell populations, requiring projection of cells into a co-embedded space to find commonality between cells in the omics space.
- Mosaic Integration: Employed when experimental designs feature various combinations of omics that create sufficient overlap across samples, such as when different sample subsets have different omics combinations measured.
Table 1: Computational Tools for Multi-Omics Integration
| Tool Name | Year | Methodology | Integration Capacity | Data Type |
|---|---|---|---|---|
| Seurat v5 | 2022 | Bridge integration | mRNA, chromatin accessibility, DNA methylation, protein | Unmatched |
| GLUE | 2022 | Graph variational autoencoders | Chromatin accessibility, DNA methylation, mRNA | Unmatched |
| MOFA+ | 2020 | Factor analysis | mRNA, DNA methylation, chromatin accessibility | Matched |
| totalVI | 2020 | Deep generative modeling | mRNA, protein | Matched |
| SCENIC+ | 2022 | Unsupervised identification model | mRNA, chromatin accessibility | Matched |
| LIGER | 2019 | Integrative non-negative matrix factorization | mRNA, DNA methylation | Unmatched |
| FigR | 2022 | Constrained optimal cell mapping | mRNA, chromatin accessibility | Matched |
| Pamona | 2021 | Manifold alignment | mRNA, chromatin accessibility | Unmatched |
Methodological Approaches and Algorithms
The computational tools for multi-omics integration employ diverse algorithmic strategies, each with distinct strengths and applications [36]:
- Matrix Factorization Methods (e.g., MOFA+): Decompose high-dimensional data into lower-dimensional representations that capture the essential factors of variation across modalities. These methods are particularly effective for identifying shared and unique sources of variation across omics layers.
- Neural Network-Based Approaches (e.g., scMVAE, DCCA, DeepMAPS): Use autoencoders and other deep learning architectures to learn non-linear representations that integrate multiple data modalities. These can capture complex interactions but typically require larger datasets for training.
- Network-Based Methods (e.g., cite-Fuse, Seurat v4): Construct biological networks that connect different omics layers, often incorporating prior knowledge about gene regulatory networks or protein-protein interactions.
- Manifold Alignment (e.g., Pamona, UnionCom): Project different omics modalities onto a common latent space while preserving the geometric structure of each individual dataset, enabling integration of unmatched data.
- Probabilistic Modeling (e.g., totalVI, MultiVI): Use statistical frameworks to account for technical noise and biological variability while integrating multiple data types.
Table 2: Quantitative Performance Metrics of Integration Methods
| Method Category | Scalability (Cell Count) | Speed (10k Cells) | Memory Usage | Key Applications |
|---|---|---|---|---|
| Matrix Factorization | 10^4-10^5 | Medium | Medium | Identifying latent factors, dimensionality reduction |
| Variational Autoencoders | 10^5-10^6 | Fast (GPU) | High | Single-cell multi-omics, missing data imputation |
| Manifold Alignment | 10^4-10^5 | Slow | Medium | Cross-species analysis, tissue atlas construction |
| Nearest Neighbor Methods | 10^5-10^6 | Fast | Low | Cell type annotation, query-to-reference mapping |
| Bayesian Models | 10^3-10^4 | Very Slow | High | Small datasets, uncertainty quantification |
Experimental Protocols for Multi-Omics Data Generation
Protocol 1: Single-Cell Multi-Omics with CITE-seq
Principle: Simultaneously measure transcriptome and surface protein expression in single cells using antibody-derived tags (ADTs) [36].
Reagents Required:
- Cell suspension (50,000-500,000 cells, viability >90%)
- Feature Barcoding reagents (10x Genomics)
- Antibody-derived tags (TotalSeq)
- Single Cell 3' GEM Kit (10x Genomics)
- Chromium Controller (10x Genomics)
- Dual Index Kit TT Set A (10x Genomics)
Procedure:
- Cell Preparation: Harvest and wash cells with PBS + 0.04% BSA. Filter through 40μm flow cytometry strainer. Count and assess viability.
- Antibody Staining: Incubate cells with TotalSeq antibody cocktail (1:100 dilution) for 30 minutes on ice. Wash twice with PBS + 0.04% BSA.
- Single Cell Partitioning: Load cells, gel beads, and partitioning oil into Chromium Chip according to 10x Genomics protocol. Target 5,000-10,000 cells.
- Library Preparation: Perform GEM generation, barcoding, and reverse transcription. Then split the reaction for separate cDNA and ADT library construction.
- Sequencing: Pool libraries and sequence on Illumina platform (Read1: 28 cycles, i7: 10 cycles, i5: 10 cycles, Read2: 90 cycles).
Quality Control Metrics:
- Cells with >500 genes and <10% mitochondrial reads
- ADT counts should correlate with expected protein expression
- Remove doublets using Scrublet or DoubletFinder
Protocol 2: Single-Cell ATAC + Gene Expression
Principle: Simultaneously profile chromatin accessibility and transcriptome in the same single cells [36].
Reagents Required:
- Nuclei isolation buffer (10mM Tris-HCl, 10mM NaCl, 3mM MgCl2, 0.1% Tween-20, 0.1% Nonidet P40, 1% BSA, 1U/μl Protector RNase Inhibant)
- Chromium Single Cell Multiome ATAC + Gene Expression Kit (10x Genomics)
- SPRIselect Reagent Kit
- E6250 Enzymatic Tagmentation Buffer
Procedure:
- Nuclei Isolation: Resuspend cells in nuclei isolation buffer. Incubate 5 minutes on ice. Wash with PBS + 0.04% BSA + RNase inhibitor.
- Transposition: Incubate nuclei with tagmentation enzyme mix for 60 minutes at 37°C. Quench with EDTA.
- GEM Generation: Combine barcoded gel beads, nuclei, and partitioning oil to form GEMs.
- Library Construction: Perform separate library preparations for ATAC and gene expression following manufacturer's protocol.
- Sequencing: Sequence on Illumina (ATAC: 50+50 paired-end; Gene Expression: 28+90 paired-end).
Quality Control Metrics:
- TSS enrichment score >5 for ATAC data
- Nucleosome banding pattern
- >1,000 genes per cell for gene expression
- FRiP score >0.2 for ATAC data
Protocol 3: Spatial Transcriptomics with Visium
Principle: Capture location-specific gene expression patterns in tissue sections [36].
Reagents Required:
- Fresh frozen or FFPE tissue sections
- Visium Spatial Gene Expression Slide & Reagents Kit (10x Genomics)
- Fixation and staining reagents
- Permeabilization enzyme
Procedure:
- Tissue Preparation: Cryosection tissue at 10μm thickness onto Visium slides. Fix with methanol.
- H&E Staining: Stain with hematoxylin and eosin for morphological context.
- Permeabilization: Optimize permeabilization time (3-30 minutes) for complete mRNA release.
- cDNA Synthesis: Perform reverse transcription with spatial barcoding.
- Library Construction: Amplify cDNA and construct sequencing libraries.
- Sequencing: Sequence on Illumina (28+150 paired-end).
Quality Control Metrics:
- >1,000 genes per spot
- >50,000 reads per spot
- Minimal tissue folding or bubbles
- Clear alignment between H&E and sequencing data
Visualization Framework for Multi-Omics Integration
Multi-Omics Integration Workflow
DBTL Cycle Enhanced with Multi-Omics Data
Research Reagent Solutions for Multi-Omics Studies
Table 3: Essential Research Reagents for Multi-Omics Integration Studies
| Reagent Category | Specific Products | Function | Application Notes |
|---|---|---|---|
| Single Cell Kits | 10x Genomics Chromium Single Cell Multiome ATAC + Gene Expression | Simultaneous profiling of chromatin accessibility and transcriptome | Optimize nuclei isolation for best results; 500-10,000 cells recommended |
| Antibody-Derived Tags | BioLegend TotalSeq, BD AbSeq | Protein surface marker detection with oligonucleotide-barcoded antibodies | Titrate antibodies carefully to minimize background; include hashing antibodies for sample multiplexing |
| Spatial Genomics | 10x Genomics Visium, Nanostring GeoMx | Location-resolved transcriptomics | FFPE or fresh frozen tissues; permeabilization time critical for mRNA capture efficiency |
| Cell Hashing Reagents | BioLegend TotalSeq-A Anti-Human Hashtag Antibodies | Sample multiplexing for single-cell experiments | Enables pooling of multiple samples, reducing batch effects and costs |
| Nucleic Acid Extraction | Qiagen AllPrep, Zymo Research Quick-DNA/RNA | Concurrent DNA and RNA isolation from same sample | Preserve molecular integrity; process samples quickly to prevent degradation |
| Library Preparation | Illumina Nextera, NEB Next Ultra | Sequencing library construction for various omics | Incorporate unique molecular identifiers (UMIs) to correct for amplification bias |
| Quality Control | Agilent Bioanalyzer, Qubit Fluorometer | Assess nucleic acid quality and quantity | RIN >8.0 for RNA studies; DNA integrity number >7 for epigenomics |
The integration of multi-omics data within the DBTL cycle represents a transformative approach to biological engineering, enabling researchers to move beyond single-layer analysis to a holistic understanding of complex biological systems. As computational methods continue to advance—particularly in machine learning and data visualization—the capacity to extract meaningful biological insights from these integrated datasets will only increase. The protocols, tools, and frameworks outlined in this whitepaper provide a foundation for researchers to implement robust multi-omics integration strategies in their own work, ultimately accelerating the pace of discovery and therapeutic development in synthetic biology and drug development. Success in this arena requires close collaboration between experimental and computational biologists, with each informing and refining the other's approaches in a truly integrated scientific workflow.
Debottlenecking Innovation: AI and Automation Strategies for an Efficient DBTL Cycle
Machine Learning and Generative AI as Predictive Design Tools
The Design-Build-Test-Learn (DBTL) cycle represents a foundational framework in synthetic biology and biological engineering, providing a systematic, iterative approach for engineering biological systems [1]. This cycle begins with the Design phase, where researchers define objectives and design biological parts using computational modeling and domain knowledge. In the Build phase, DNA constructs are synthesized and assembled into vectors for introduction into characterization systems. The Test phase experimentally measures the performance of these engineered constructs, while the Learn phase analyzes collected data to inform subsequent design rounds [2].
Traditionally, the DBTL cycle has relied heavily on empirical iteration, with the Build-Test portions often creating bottlenecks despite automation advances [2] [1]. However, the integration of artificial intelligence is fundamentally transforming this paradigm. Rather than treating "Learning" as the final step that follows physical testing, machine learning (ML) and generative AI now enable predictive design that can precede the Design phase itself. This evolution shifts the traditional DBTL cycle to an "LDBT" approach, where Learning based on large datasets and foundational models informs the initial Design, potentially reducing the need for multiple iterative cycles [2].
This technical guide explores how ML and generative AI serve as predictive design tools within this evolving framework, focusing on applications across protein engineering, drug discovery, and strain development for researchers and drug development professionals.
AI and ML Fundamentals for Predictive Design
Machine learning algorithms excel at identifying complex patterns within high-dimensional biological data that are often imperceptible to human researchers or traditional computational methods. These capabilities are particularly valuable for navigating the intricate relationship between a protein's sequence, structure, and function—a central challenge in biological design [2].
Key Algorithmic Approaches
Protein Language Models: Models such as ESM and ProGen are trained on evolutionary relationships embedded in millions of protein sequences [2]. These models capture long-range dependencies within amino acid sequences, enabling zero-shot prediction of protein functions, beneficial mutations, and solvent-accessible regions without additional training [2]. For example, ESM has demonstrated particular efficacy in predicting functional antibody sequences [2].
Structure-Based Design Tools: ProteinMPNN represents a structure-based deep learning approach that takes entire protein backbones as input and generates sequences likely to fold into those structures [2]. When combined with structure assessment tools like AlphaFold or RoseTTAFold, this approach has demonstrated nearly a 10-fold increase in design success rates compared to traditional methods [2].
Generative AI Models: Generative adversarial networks (GANs) and transformer architectures create novel molecular structures with desired properties, exploring chemical spaces beyond human intuition [37] [38]. These models can be trained on vast chemical libraries to generate novel compounds optimized for specific therapeutic targets or biological functions [37] [39]. For instance, GPT-based molecular generators like ChemSpaceAL enable protein-specific molecule generation, creating virtual screening libraries tailored to particular binding sites [37].
Quantitative Performance of ML in Drug Discovery
Table 1: Market Adoption of Machine Learning in Drug Discovery (2024)
| Segment | Market Share (%) | Key Drivers |
|---|---|---|
| By Application Stage | ||
| Lead Optimization | ~30% | AI/ML-driven optimization of drug efficiency, safety, and development timelines [40] |
| Clinical Trial Design & Recruitment | Fastest growing | Personalized trial models and biomarker-based stratification from patient data [40] |
| By Algorithm Type | ||
| Supervised Learning | ~40% | Ability to estimate drug activity and properties using labeled datasets [40] |
| Deep Learning | Fastest growing | Structure-based predictions and AlphaFold applications in protein modeling [40] |
| By Therapeutic Area | ||
| Oncology | ~45% | Rising cancer prevalence driving demand for personalized therapies [40] |
| Neurological Disorders | Fastest growing | Increasing incidence of Alzheimer's and Parkinson's requiring novel treatments [40] |
Table 2: AI-Accelerated Drug Discovery Timelines and Success Rates
| Metric | Traditional Approach | AI-Enhanced Approach |
|---|---|---|
| Initial Drug Design Phase | 2-5 years | 6-18 months [41] |
| Candidate Identification | Months to years | Days to weeks [38] |
| Cost per Developed Drug | $2.6 billion [42] | 25-50% reduction [37] |
| Clinical Trial Success Rate | 7.9% (Phase I to approval) [42] | Improved patient stratification and endpoint prediction [38] |
Experimental Protocols for AI-Driven Biological Design
Cell-Free Protein Synthesis for Rapid Testing
Purpose: To rapidly express and test AI-designed protein variants without the constraints of cellular transformation and growth [2].
Methodology:
- Lysate Preparation: Create cell lysates from appropriate expression systems (E. coli, wheat germ, or insect cells) containing transcriptional and translational machinery [2].
- DNA Template Design: Synthesize DNA templates encoding AI-designed protein variants without cloning steps.
- Reaction Assembly: Combine DNA templates with cell-free reaction mixtures containing amino acids, energy sources, and cofactors.
- Expression and Analysis: Incubate reactions at 30-37°C for 4-24 hours, then analyze protein expression and function [2].
Key Applications:
- Ultra-high-throughput stability mapping of 776,000 protein variants [2]
- Screening 500 computationally designed antimicrobial peptides [2]
- Pathway prototyping through iPROBE (in vitro prototyping and rapid optimization of biosynthetic enzymes) [2]
Advantages:
- Rapid protein production (>1 g/L in <4 hours) [2]
- Bypass of cellular toxicity limitations [2]
- Scalability from picoliter to kiloliter scales [2]
- Direct integration with liquid handling robots and microfluidics [2]
In Vitro to In Vivo Translation for Metabolic Engineering
Purpose: To leverage cell-free systems for pathway prototyping before implementing designs in living production hosts [11].
Methodology (as demonstrated for dopamine production in E. coli):
- In Vitro Pathway Validation:
- Express individual enzymes (HpaBC and Ddc) in cell-free transcription-translation systems [11]
- Test different relative expression levels to determine optimal enzyme ratios
- Measure dopamine production from precursor L-tyrosine
In Vivo Implementation:
- Translate optimal expression ratios to production host using RBS engineering
- Design RBS libraries with varying Shine-Dalgarno sequences
- Assemble constructs using automated molecular cloning workflows
- Transform into engineered E. coli FUS4.T2 with enhanced L-tyrosine production [11]
Strain Analysis:
- Cultivate production strains in minimal medium with appropriate carbon sources
- Measure dopamine titers using HPLC or colorimetric assays
- Compare production levels to state-of-the-art benchmarks [11]
Results: This knowledge-driven DBTL approach achieved dopamine production of 69.03 ± 1.2 mg/L (34.34 ± 0.59 mg/g biomass), representing a 2.6 to 6.6-fold improvement over previous methods [11].
Generative AI for Undruggable Target Development
Purpose: To design therapeutic antibodies against challenging targets like GPCRs and ion channels [41].
Methodology:
- Target Analysis:
- Collect structural and sequence data for target proteins
- Identify potential binding epitopes, even those only a few amino acids wide
Epitope-Specific Library Generation:
- Train generative AI models on antibody-antigen interaction datasets
- Generate diverse antibody sequences targeting specific epitopes
- Optimize properties like binding affinity and specificity
In Silico Screening:
- Predict binding affinities using deep learning models
- Perform multi-parameter clustering to select lead candidates
- Utilize deep mutational scanning to optimize antibody characteristics [41]
Experimental Validation:
- Express and purify selected antibody candidates
- Measure binding kinetics and functional activity
- Assess specificity against related targets
Advantages Over Traditional Methods:
- Higher predictive accuracy for finding functional binders [41]
- Ability to target previously inaccessible binding sites [41]
- Condensed discovery timeline from years to approximately six months [41]
Visualization of AI-Enhanced Workflows
AI-Enhanced LDBT Cycle Versus Traditional DBTL
Predictive Design Workflow Integrating AI and Experimental Validation
The Scientist's Toolkit: Essential Research Reagents and Platforms
Table 3: Key Research Reagent Solutions for AI-Driven Biological Design
| Category | Specific Tools/Platforms | Function | Application Examples |
|---|---|---|---|
| AI/ML Software | ESM, ProGen (Protein Language Models) | Zero-shot prediction of protein function and mutations | Predicting beneficial mutations for enzyme engineering [2] |
| ProteinMPNN, MutCompute (Structure-Based Design) | Sequence design for specific protein backbones | Designing stable hydrolase variants for PET depolymerization [2] | |
| AlphaFold, RoseTTAFold (Structure Prediction) | Protein structure prediction from sequence | Determining structures of G6Pase-α and G6Pase-β with active sites [37] | |
| Experimental Systems | Cell-Free Expression Platforms | Rapid in vitro protein synthesis without cloning | Expressing 776,000 protein variants for stability mapping [2] |
| pET, pJNTN Plasmid Systems | Vector systems for heterologous gene expression | Dopamine production pathway assembly in E. coli [11] | |
| pSEVA261 Backbone | Medium-low copy number plasmid for biosensors | Reducing background signal in PFAS biosensor development [43] | |
| Automation & Screening | Droplet Microfluidics (DropAI) | Ultra-high-throughput screening of reactions | Screening >100,000 picoliter-scale reactions [2] |
| Liquid Handling Robots | Automated reagent distribution and assembly | Enabling high-throughput molecular cloning workflows [1] | |
| Specialized Reagents | Ribosome Binding Site (RBS) Libraries | Fine-tuning gene expression in synthetic pathways | Optimizing dopamine pathway enzyme ratios [11] |
| LuxCDEAB Operon Reporter | Bioluminescence-based promoter activity sensing | Developing PFAS biosensors with split operon design [43] |
The integration of machine learning and generative AI into biological engineering represents a paradigm shift from empirical iteration to predictive design. The traditional DBTL cycle, while effective, often requires multiple time-consuming and resource-intensive iterations to achieve desired biological functions. The emerging LDBT framework, where Learning precedes Design through sophisticated AI models, demonstrates potential to compress development timelines from years to months while substantially reducing costs [2] [37].
As these technologies mature, several key trends are emerging: the rise of foundation models for biology that capture fundamental principles of biomolecular interactions, increased integration of automated experimental systems for rapid validation, and growing application to previously intractable challenges like undruggable targets [41]. For researchers and drug development professionals, mastery of these predictive design tools is becoming essential for maintaining competitive advantage and addressing increasingly complex biological engineering challenges.
The future of biological design lies in the seamless integration of computational prediction and experimental validation, creating a virtuous cycle where AI models generate designs, high-throughput systems test them, and resulting data further refine the models. This synergistic approach promises to accelerate the development of novel therapeutics, biosensors, and sustainable biomanufacturing platforms, ultimately transforming how we engineer biological systems to address global challenges.
The field of biological engineering research is fundamentally guided by the Design-Build-Test-Learn (DBTL) cycle, an iterative framework that transforms biological design into tangible outcomes [44]. In traditional research settings, executing this cycle is often hampered by manual, low-throughput processes that are time-consuming, costly, and prone to variability. Biofoundries represent a paradigm shift, emerging as integrated, automated facilities designed to accelerate and standardize synthetic biology applications by facilitating high-throughput execution of the DBTL cycle [45]. These facilities strategically integrate robotics, advanced analytical instruments, and computational analytics to streamline and expedite the entire synthetic biology workflow [44]. This whitepaper examines the transformative role of biofoundries, with a specific focus on how they bring unprecedented standardization and scale to the critical "Build" and "Test" phases, thereby enhancing the reproducibility, efficiency, and overall impact of biological engineering research for scientists and drug development professionals.
The DBTL Framework in Biofoundry Operations
At its core, a biofoundry operates by implementing the DBTL cycle at scale [44] [46]. The cycle begins with the Design (D) phase, where computational tools are used to design genetic sequences or biological circuits. This is followed by the Build (B) phase, which involves the automated, high-throughput construction of the designed biological components. The Test (T) phase then employs high-throughput screening to characterize the constructed systems. Finally, in the Learn (L) phase, data from the test phase are analyzed and used to inform the next design iteration, thus closing the loop [44]. To manage the complexity of these automated processes, a structured abstraction hierarchy has been proposed, organizing biofoundry activities into four interoperable levels: Project, Service/Capability, Workflow, and Unit Operation [47] [48]. This framework is crucial for achieving modular, reproducible, and scalable experimental workflows.
The following diagram illustrates the continuous, iterative nature of the DBTL cycle, which serves as the core operational principle of a biofoundry.
Standardizing the Build Phase
The Build phase is where designed genetic constructs are physically synthesized and assembled. In a biofoundry, this process is transformed from a manual artisanal practice into a standardized, high-throughput operation.
Core Principles and Architectural Automation
Standardization in the Build phase is achieved through automation and modular workflows. The goal is to convert a biological design into a physical reality—such as DNA, RNA, or an engineered strain—reproducibly and at scale [49]. Biofoundries employ varying degrees of laboratory automation, which can be architecturally classified into several configurations [45]:
- Single Robot-Single Workflow (SR-SW): A single robot, such as a liquid handling system, is dedicated to one specific workflow.
- Multi Robot-Single Workflow (MR-SW): Multiple robots are linked in a fixed sequence to execute a single, more complex workflow.
- Multi Robot-Multi Workflow (MR-MW): Multiple robots are reconfigured to support different workflows as needed.
- Modular Cell Workstation (MCW): This highly flexible architecture uses mobile robots and standardized module interfaces to enable dynamic, parallel execution of multiple workflows.
The essence of standardization lies in deconstructing complex protocols into smaller, reusable Unit Operations, which are the smallest units of experimental tasks performed by a single piece of equipment or software [47] [48]. For example, a "DNA Oligomer Assembly" workflow can be broken down into a sequence of 14 distinct unit operations, such as liquid transfer, centrifugation, and thermocycling [47].
Key Build Phase Workflows and Protocols
Two critical workflows exemplify the standardized Build phase in action:
Automated DNA Assembly: This workflow involves the construction of genetic circuits from smaller DNA parts. A typical protocol, executable on platforms like Opentrons OT-2 or BioAutomat, leverages software such as j5 or AssemblyTron to automate the assembly design and liquid handling instructions [44] [45]. The process involves:
- Liquid Handling for Reaction Setup: A robot precisely dispenses DNA parts, assembly master mix, and enzymes into a microplate (e.g., 96-well format) [47].
- Thermocycling: The plate is transferred to a thermocycler for the specific incubation steps required by the assembly method (e.g., Golden Gate, Gibson Assembly).
- Transformation: The assembled product is transformed into competent E. coli cells using a high-throughput electroporator.
- Plating and Colony Picking: Cells are plated on selective agar plates, and after incubation, a colony picker robot selects successful transformants for liquid culture in a deep-well plate [45].
Strain Engineering for Microbial Cell Factories: This workflow focuses on introducing genetic modifications into a production chassis (e.g., E. coli, S. cerevisiae). A standard methodology using CRISPR-Cas9 involves:
- Oligo Synthesis: Design and synthesis of gRNA(s) and donor DNA templates.
- Vector Preparation: Preparation of the CRISPR plasmid(s) through automated DNA assembly or direct synthesis.
- High-Throughput Transformation: Introduction of the CRISPR system into the host cells.
- Screening: Automated selection and screening of successful edits, often via antibiotic selection or fluorescence-activated cell sorting (FACS) [45]. Tools like MACBETH demonstrate this with a multiplex automated base-editing method for Corynebacterium glutamicum [45].
The Scientist's Toolkit: Essential Reagents for the Build Phase
Table 1: Key Research Reagent Solutions for the Build Phase
| Reagent/Material | Function | Example Application |
|---|---|---|
| DNA Parts/ Oligonucleotides | Basic building blocks for gene synthesis and assembly. | Modular assembly of genetic circuits; CRISPR gRNA templates. |
| Assembly Master Mix | Enzyme mix containing DNA ligase and/or polymerase for seamless DNA assembly. | Golden Gate and Gibson Assembly reactions. |
| CRISPR-Cas9 System | Plasmid(s) expressing Cas9 protein and guide RNA for precise genome editing. | Knock-in, knock-out, and base editing in microbial and mammalian cells. |
| Competent E. coli Cells | Chemically or electro-competent cells for plasmid propagation. | Transformation of assembled DNA constructs for amplification and verification. |
| Selection Antibiotics | Adds selective pressure to maintain plasmids or select for edited cells. | Added to growth media (agar or liquid) post-transformation. |
| Lysis Reagents | Chemical or enzymatic mixes to break open cells for DNA extraction. | Automated preparation of DNA samples for sequence verification. |
Scaling the Test Phase
The Test phase is where the functionality and performance of the built constructs are rigorously evaluated. Biofoundries scale this phase through high-throughput analytical techniques and automated data capture.
Core Principles and High-Throughput Analytics
The primary objective of the Test phase is the high-throughput screening and characterization of engineered biological systems to validate their function, safety, and performance [49]. Scaling is achieved by miniaturizing assays into microplate formats (96, 384, or 1536 wells) and using automated instruments for rapid, parallel analysis. The integration of automation and data management systems is critical for streamlining sample tracking and result interpretation [49]. Key technologies include:
- Microplate Readers: For absorbance, fluorescence, and luminescence measurements to quantify reporter gene expression, cell density, and metabolic activity.
- Flow Cytometers: For single-cell analysis, enabling the characterization of population heterogeneity and the sorting of high-performing cells.
- Liquid Chromatography-Mass Spectrometry (LC-MS): For quantifying small molecules, such as target metabolites or products, in culture supernatants [45].
- Next-Generation Sequencing (NGS): For validating genetic constructs and checking for unintended mutations at scale.
Key Test Phase Workflows and Protocols
Standardized Test workflows are essential for generating reproducible and comparable data:
High-Throughput Screening of Metabolite Production: This workflow is used to identify top-performing engineered strains from a library. A standard protocol involves:
- Inoculation and Cultivation: Using a liquid handler, picked colonies are inoculated into deep-well plates containing growth medium and incubated in a shaking incubator.
- Sample Extraction: After a defined growth period, a portion of the culture is automatically transferred to a new microplate. Cells are pelleted via centrifugation, and the supernatant is moved to an analysis plate.
- Analysis: The supernatant is analyzed directly or after derivatization. For example, LC-MS with an autosampler is used to precisely quantify the titer of the target molecule, such as a biofuel precursor or therapeutic compound [45].
Growth-Based Phenotypic Assays: This workflow assesses the impact of genetic modifications on cell fitness and overall phenotype. A typical methodology includes:
- Automated Dilution and Dispensing: Cultures are automatically diluted to a standard optical density (OD) and dispensed into assay plates.
- Continuous Kinetic Monitoring: Plates are loaded into a plate reader that takes periodic OD600 (for biomass) and fluorescence (for reporter expression) measurements over 12-48 hours.
- Data Processing: Growth curves (OD over time) and product formation curves (fluorescence over time) are automatically generated. Parameters like maximum growth rate and final product yield are extracted for analysis [50].
The Scientist's Toolkit: Essential Reagents for the Test Phase
Table 2: Key Research Reagent Solutions for the Test Phase
| Reagent/Material | Function | Example Application |
|---|---|---|
| Defined Growth Media | Provides consistent nutrients for cell growth and product formation. | High-throughput micro-fermentations; phenotyping assays. |
| Fluorescent Reporters/Dyes | Molecules that emit light upon binding or enzymatic action. | Quantifying promoter activity; assessing cell viability. |
| Enzyme Activity Assay Kits | Pre-formulated reagent mixes for specific enzymatic reactions. | Screening enzyme libraries for improved catalysts. |
| Metabolite Standards | Pure chemical standards for target molecules. | Calibrating LC-MS for accurate quantification of product titer. |
| Antibodies & Detection Reagents | For immunoassays to detect and quantify specific proteins. | ELISA-based screening of antibody or protein production. |
| Sample Preparation Kits | Reagents for automated nucleic acid or protein purification. | Preparing sequencing libraries or protein samples for analysis. |
Quantitative Impact and Future Perspectives
The implementation of standardized and scaled Build and Test phases delivers measurable improvements in research and development productivity.
Table 3: Quantitative Impact of Biofoundry Implementation
| Metric | Traditional Manual Process | Biofoundry-Enabled Process | Source / Example |
|---|---|---|---|
| Screening Throughput | ~10,000 yeast strains per year | 20,000 strains per day | Lesaffre Biofoundry [50] |
| Project Timeline | 5-10 years | 6-12 months | Lesaffre Genetic Improvement [50] |
| DNA Assembly & Strain Construction | Weeks to months for multiple constructs | 1.2 Mb DNA, 215 strains across 5 species in 90 days | DARPA Battle Challenge [44] |
| Market Valuation | N/A | USD 1.15 Billion (2024) Projected USD 6.28 Billion (2033) | Biofoundry-as-a-Service Market [49] |
The future of biofoundries is intrinsically linked to advances in Artificial Intelligence (AI) and machine learning (ML). AI is transforming biofoundries by enhancing the precision of predictions in the Design phase and actively learning from Build and Test data to guide subsequent iterations [45]. We are witnessing the emergence of self-driving labs, where the DBTL cycle is fully automated and coupled with AI-driven experimental planning, requiring minimal human intervention [44] [45]. Furthermore, global initiatives like the Global Biofoundry Alliance (GBA), which now includes over 30 member organizations, are crucial for addressing shared challenges, promoting interoperability through standardized frameworks, and fostering a collaborative ecosystem to accelerate synthetic biology innovation [44] [47]. This collaborative, data-driven future promises to further compress development timelines and unlock new possibilities in therapeutic development and sustainable biomanufacturing.
Smart Sensing and Real-Time Data Acquisition for Dynamic Process Control
In the context of biological engineering, the Design-Build-Test-Learn (DBTL) cycle provides a systematic framework for engineering biological systems [1]. This cyclical process involves designing biological parts, building DNA constructs, testing their performance in vivo or in vitro, and learning from the data to inform the next design iteration [2]. The integration of smart sensing technologies and real-time data acquisition creates a transformative opportunity to accelerate this cycle, enabling dynamic process control that enhances both the efficiency and output of bioengineering research and production.
Smart sensors, equipped with embedded processing and connectivity capabilities, provide a technological foundation for continuous monitoring of critical process variables [51] [52]. When applied within the DBTL framework, these sensors generate the high-resolution, time-series data essential for optimizing bioprocesses, from laboratory-scale pathway prototyping to industrial-scale fermentation. This technical guide explores the integration of smart sensing, data acquisition, and dynamic control within the DBTL paradigm, providing methodologies and resources for researchers and drug development professionals.
The DBTL Cycle: Foundation for Engineering Biology
The DBTL cycle is a core engineering mantra in synthetic biology [2] [1]. Each phase plays a distinct role:
- Design: Researchers define objectives and design biological parts or systems using computational models and domain knowledge.
- Build: DNA constructs are assembled and introduced into characterization systems (e.g., microbial chassis or cell-free systems).
- Test: Engineered constructs are experimentally measured for performance, a phase greatly enhanced by smart sensor data acquisition.
- Learn: Data from testing is analyzed to refine understanding and inform subsequent design rounds.
Recent proposals suggest a paradigm shift to LDBT (Learn-Design-Build-Test), where machine learning models trained on large biological datasets precede the design phase, potentially enabling functional solutions in a single cycle [2]. This reordering leverages zero-shot predictions from advanced algorithms, though it still requires physical validation through the Build and Test phases.
Figure 1: The LDBT Cycle Enhanced by Real-Time Data. This adapted cycle shows how machine learning (Learn) precedes Design, with smart sensor data and dynamic control creating an integrated workflow.
Smart Sensing Technology for Bioprocess Monitoring
Evolution and Capabilities
Smart sensors represent the evolutionary pinnacle of sensor technology, featuring enhanced capabilities including connectivity, embedded processing, and adaptability to diverse biological environments [52]. Unlike traditional sensors that simply collect data, smart sensors can interpret and analyze information on the spot, providing a wealth of insights that contribute to improved decision-making within research and production settings.
Key Sensor Types for Bioprocess Control
In biological process control, several sensor types are critical for monitoring key variables:
- Temperature Sensors: Monitor and maintain optimal growth conditions for microbial hosts or enzymatic activity.
- Pressure Sensors: Ensure integrity of bioreactor systems and monitor gas evolution in anaerobic processes.
- Chemical/Gas Sensors: Track dissolved oxygen, carbon dioxide, pH, and metabolic byproducts in real-time.
- Optical Density Sensors: Provide real-time biomass measurements for tracking culture growth phases.
- Metabolite Sensors: Enable monitoring of specific pathway intermediates or end products.
Quantitative Comparison of Sensor Technologies
Table 1: Performance Characteristics of Smart Sensors in Bioprocess Monitoring
| Sensor Type | Measured Variable | Accuracy | Response Time | Integration Complexity |
|---|---|---|---|---|
| Optical Density | Biomass concentration | ±2% of reading | <1 second | Low |
| pH | Hydrogen ion activity | ±0.01 pH | 2-5 seconds | Medium |
| Dissolved O₂ | Oxygen concentration | ±1% of reading | 5-10 seconds | High |
| CO₂ | Carbon dioxide levels | ±2% of reading | 10-30 seconds | Medium |
| Metabolite | Specific compounds | Varies by analyte | 30-60 seconds | High |
Real-Time Data Acquisition Systems
Architecture and Components
Real-time data acquisition systems form the bridge between physical sensors and computational analysis. These systems typically comprise:
- Sensor Interface Modules: Condition signals from analog sensors to digital data.
- Data Loggers/Controllers: Aggregate multiple data streams with precise timestamps.
- Communication Protocols: Enable data transfer via wired (Ethernet, USB) or wireless (Wi-Fi, Bluetooth) interfaces.
- Edge Processing Units: Perform preliminary data analysis and filtering at the source.
Significance for DBTL Cycles
The immediacy of real-time data acquisition enables proactive responses to changing bioprocess conditions [51] [52]. In a DBTL context, this means researchers can:
- Monitor pathway performance during the Test phase without manual sampling
- Detect early signs of culture stress or metabolic burden
- Make informed decisions about process adjustments or termination
- Collect continuous data streams for model training in the Learn phase
Integrating Sensing with Dynamic Process Control
Adaptive Control Systems
Smart sensors enable the development of adaptive control systems that dynamically respond to changes in bioprocess conditions [52]. These systems can adjust parameters in real-time to optimize performance, creating a responsive bio-manufacturing environment.
Table 2: Control Strategies Enhanced by Real-Time Sensing
| Control Strategy | Key Input Sensors | Typical Actuators | Application in DBTL |
|---|---|---|---|
| Feedback Control | pH, DO, temperature | Acid/base pumps, heater/cooler, aeration | Maintain optimal testing conditions |
| Feedforward Control | Substrate, metabolite | Nutrient feed pumps | Anticipate metabolic shifts |
| Model Predictive Control | Multi-parameter inputs | All available actuators | Implement learned models in next Test cycle |
| Fuzzy Logic Control | Pattern recognition from multiple sensors | Adaptive system parameters | Handle complex, non-linear bioprocesses |
Experimental Workflow for Sensor-Enabled Bioprocess Optimization
Figure 2: Experimental workflow for sensor-enabled bioprocess optimization, showing the integration of physical and computational elements.
Detailed Experimental Protocol: Sensor-Enabled Bioprocess Characterization
Materials and Equipment
Research Reagent Solutions and Essential Materials
Table 3: Key Research Reagents and Materials for Sensor-Enabled Bioprocess Monitoring
| Item | Function/Application | Specifications |
|---|---|---|
| Minimal Medium | Defined growth medium for microbial cultures | 20 g/L glucose, 10% 2xTY, phosphate buffer, trace elements [11] |
| Trace Element Stock | Provides essential micronutrients | FeCl₃, ZnSO₄, MnSO₄, CuSO₄, CoCl₂, CaCl₂, MgSO₄, sodium citrate [11] |
| Phosphate Buffer | Maintains pH stability | 50 mM, pH 7.0 [11] |
| Antibiotic Solutions | Selective pressure for plasmid maintenance | Ampicillin (100 µg/mL), Kanamycin (50 µg/mL) [11] |
| Induction Solution | Triggers expression of pathway genes | IPTG (1 mM final concentration) [11] |
| Calibration Standards | Sensor validation and calibration | pH buffers, certified gas mixtures, analyte standards |
Step-by-Step Methodology
System Setup and Sterilization
- Assemble bioreactor system with integrated smart sensors for temperature, pH, dissolved oxygen, and optical density.
- Sterilize in-place or autoclave sensor probes according to manufacturer specifications.
- Validate sensor functionality and communication protocols pre-inoculation.
Sensor Calibration
- Calibrate pH sensor using standard buffers at pH 4.0, 7.0, and 10.0.
- Calibrate dissolved oxygen sensor using two-point method (0% and 100% saturation).
- Establish baseline for optical density sensor with sterile medium.
- Verify calibration by measuring known standards.
Process Monitoring and Data Acquisition
- Inoculate bioreactor with pre-culture of engineered production strain.
- Initiate continuous data acquisition from all sensors at 30-second intervals.
- Monitor key parameters throughout batch, fed-batch, or continuous process.
- Implement automated control loops for critical parameters (e.g., temperature, pH).
Dynamic Process Adjustments
- Trigger nutrient feeding based on real-time dissolved oxygen spikes.
- Induce pathway expression at specific growth phases identified by optical density.
- Adjust aeration based on oxygen uptake rates calculated in real-time.
- Sample for offline validation of sensor readings and metabolite analysis.
Data Integration and Analysis
- Correlate real-time sensor data with endpoint analytical measurements.
- Identify process anomalies or deviations from expected patterns.
- Extract features for model training in the Learn phase of DBTL.
- Document all process events and interventions with precise timestamps.
Case Study: DBTL-Optimized Dopamine Production
A recent implementation of the knowledge-driven DBTL cycle for dopamine production in E. coli demonstrates the value of integrated data acquisition [11]. Researchers developed a highly efficient dopamine production strain through the following approach:
In Vitro Pathway Prototyping: Used cell-free transcription-translation systems to test different enzyme expression levels before in vivo implementation.
RBS Library Construction: Engineered a library of ribosome binding site variants to fine-tune expression of HpaBC and Ddc enzymes.
High-Throughput Screening: Employed automated cultivation and analytics to identify optimal strain variants.
Real-Time Bioprocess Monitoring: Implemented sensor arrays to track dopamine production kinetics in bioreactors.
This approach resulted in a dopamine production strain achieving 69.03 ± 1.2 mg/L, a 2.6 to 6.6-fold improvement over previous state-of-the-art in vivo production [11].
Data Management and Reproducibility
Systems like BioWes provide solutions for managing experimental data and metadata to support reproducibility in data-intensive biological research [53]. These platforms:
- Standardize terminology and protocol design
- Link experimental data with complete process descriptions
- Enable data sharing while maintaining sensitive information locally
- Support protocol evolution and version control
Proper data management ensures that sensor data acquired during DBTL cycles remains traceable, reproducible, and available for future learning phases and meta-analyses.
Future Directions and Challenges
Emerging Trends
The convergence of smart sensor technology with machine learning and cloud computing will further transform bioprocess control [2] [52]. Specific advances include:
- AI-Enhanced Sensor Fusion: Combining multiple sensor inputs with machine learning models to predict difficult-to-measure variables.
- Digital Twins: Creating virtual replicas of bioprocesses that update in real-time with sensor data.
- Edge AI: Performing real-time inference for process control directly on sensor modules.
Implementation Challenges
Despite the promise, several challenges remain:
- Data Security: Protecting sensitive process data and intellectual property [51].
- Interoperability: Ensuring sensors from different manufacturers can integrate seamlessly [52].
- Skill Gaps: Requiring interdisciplinary expertise in both biology and data science.
- Cost-Benefit Analysis: Justifying capital investment in advanced sensor systems.
Smart sensing and real-time data acquisition represent fundamental enabling technologies for advancing the DBTL cycle in biological engineering. By providing continuous, high-resolution insights into bioprocess performance, these systems transform the Test phase from a bottleneck to a rich source of actionable data. This enhancement accelerates the entire engineering cycle, enabling more rapid development of optimized microbial strains and bioprocesses. As these technologies continue to converge with machine learning and automation, they promise to further solidify the DBTL framework as a powerful paradigm for biological design, ultimately advancing therapeutic development and sustainable biomanufacturing.
The Design-Build-Test-Learn (DBTL) cycle is a foundational framework in synthetic biology for the systematic engineering of biological systems [1]. Traditional DBTL cycles often begin with limited prior knowledge, requiring multiple iterative rounds that consume significant time and resources [24]. A transformative approach emerging in the field is the "knowledge-driven" DBTL cycle, which incorporates upstream in vitro investigations to create a mechanistic understanding of biological systems before embarking on in vivo engineering [24]. This methodology strategically uses cell-free transcription-translation (TX-TL) systems and computational modeling to de-risk the initial design phase, enabling more informed predictions about cellular behavior and significantly accelerating the development of high-performance production strains [24] [2].
This whitepaper explores the pivotal role of the knowledge-driven DBTL cycle within modern biological engineering research. Using a case study of microbial dopamine production, we will detail how this approach successfully bridged the gap between in vitro prototyping and in vivo implementation. Furthermore, we will examine how the integration of machine learning and automated biofoundries is evolving the traditional DBTL paradigm into more efficient sequences, such as the LDBT (Learn-Design-Build-Test) cycle, where predictive models guide design from the outset [2] [3]. This structured, data-centric framework is proving essential for advancing strain engineering for therapeutic molecules, sustainable chemicals, and novel biomaterials.
Core Principles of the Knowledge-Driven Workflow
The knowledge-driven DBTL cycle distinguishes itself from traditional approaches through its foundational strategy. It replaces initial trial-and-error with mechanistic understanding gained from upstream experiments. A key enabler of this approach is the use of cell-free systems, such as crude cell lysates, which provide a flexible environment for prototyping genetic circuits and metabolic pathways without the complexities of a living cell [24] [2]. These systems allow researchers to test enzyme expression levels, pathway fluxes, and system interactions rapidly and under controlled conditions, bypassing cellular constraints like membrane permeability and internal regulation [24].
The workflow translates insights from in vitro experiments directly into in vivo strain engineering. Findings on relative enzyme expression levels and co-factor requirements from cell-free systems inform the precise tuning of genetic parts for the living host [24]. This translation is often achieved through high-throughput techniques like Ribosome Binding Site (RBS) engineering to fine-tune translation initiation rates [24]. The entire process is increasingly powered by machine learning models that are trained on the data generated from both in vitro and in vivo testing. These models learn the complex relationships between DNA sequence design, expression levels, and final product titer, allowing for increasingly smarter designs in subsequent cycles [2] [54].
The Evolving DBTL Paradigm: From DBTL to LDBT
The integration of powerful machine learning is prompting a re-evaluation of the traditional cycle. A proposed new paradigm, LDBT (Learn-Design-Build-Test), places "Learn" at the beginning [2] [3]. In this model, the cycle starts with machine learning models that have been pre-trained on vast biological datasets. These models can make "zero-shot" predictions—designing functional biological parts without needing iterative experimental data from the specific system [2]. This learning-first approach leverages artificial intelligence to design constructs that are more likely to succeed from the outset, potentially reducing the number of DBTL cycles required and accelerating the path to a high-performing strain [2] [54].
Case Study: Developing an Efficient Dopamine Production Strain
A 2025 study on the microbial production of dopamine provides a compelling demonstration of the knowledge-driven DBTL cycle in action [24]. Dopamine is an organic compound with critical applications in emergency medicine, cancer diagnosis, and wastewater treatment [24]. The goal was to engineer an E. coli strain capable of efficient de novo dopamine synthesis, overcoming the limitations of traditional chemical synthesis methods which are environmentally harmful and resource-intensive [24].
Experimental Workflow and Pathway Design
The project involved a meticulously planned workflow that seamlessly integrated in vitro and in vivo stages. The dopamine biosynthetic pathway was constructed in E. coli using a two-step process starting from the precursor L-tyrosine. First, the native E. coli enzyme 4-hydroxyphenylacetate 3-monooxygenase (HpaBC) converts L-tyrosine to L-DOPA. Subsequently, a heterologous L-DOPA decarboxylase (Ddc) from Pseudomonas putida catalyzes the formation of dopamine [24]. The host strain was first engineered for high L-tyrosine production by depleting the transcriptional repressor TyrR and mutating the feedback inhibition of the TyrA enzyme [24].
The knowledge-driven approach was implemented by first testing the expression and functionality of the pathway enzymes (HpaBC and Ddc) in a crude cell lysate system derived from the production host. This in vitro step allowed the researchers to study the pathway mechanics and identify optimal relative expression levels for the enzymes without the complications of cellular metabolism [24]. The insights gained about required expression levels were then directly translated to the in vivo environment through high-throughput RBS engineering, which allowed for precise fine-tuning of the translation initiation rates for each gene in the pathway [24].
Key Research Reagents and Solutions
Table 1: Essential research reagents and materials used in the knowledge-driven DBTL workflow for dopamine strain engineering.
| Reagent/Material | Function/Description | Application in Workflow |
|---|---|---|
| Crude Cell Lysate | Transcription-translation machinery extracted from E. coli; provides metabolites and energy equivalents [24]. | In vitro pathway prototyping and enzyme activity testing. |
| RBS (Ribosome Binding Site) Library | A collection of DNA sequences with varying Shine-Dalgarno sequences to modulate translation initiation rates [24]. | Fine-tuning relative expression levels of HpaBC and Ddc genes in vivo. |
| L-Tyrosine | Aromatic amino acid precursor for the dopamine biosynthetic pathway [24]. | Substrate for the pathway; supplemented in fermentation media. |
| HpaBC Gene | Encodes 4-hydroxyphenylacetate 3-monooxygenase; converts L-tyrosine to L-DOPA [24]. | Key enzymatic component of the heterologous dopamine pathway. |
| Ddc Gene | Encodes L-DOPA decarboxylase from Pseudomonas putida; converts L-DOPA to dopamine [24]. | Final enzymatic step in the heterologous dopamine pathway. |
| Specialized Minimal Medium | Defined growth medium with controlled carbon source (glucose), salts, and trace elements [24]. | High-throughput cultivation and fermentation of engineered strains. |
Quantitative Results and Performance Metrics
The knowledge-driven approach yielded a highly efficient dopamine production strain. The optimized strain achieved a dopamine titer of 69.03 ± 1.2 mg/L, which corresponds to a yield of 34.34 ± 0.59 mg/g biomass [24]. This represents a substantial improvement over previous state-of-the-art in vivo dopamine production methods, with performance enhancements of 2.6-fold in titer and 6.6-fold in yield [24]. A critical finding from the learning phase was the demonstrated impact of the GC content in the Shine-Dalgarno sequence on the strength of the RBS and, consequently, on the final dopamine production levels [24].
Table 2: Summary of quantitative outcomes from the dopamine production strain engineering campaign.
| Performance Metric | Result from Knowledge-Driven DBTL | Fold Improvement Over Previous State-of-the-Art |
|---|---|---|
| Dopamine Titer | 69.03 ± 1.2 mg/L | 2.6-fold |
| Specific Yield | 34.34 ± 0.59 mg/g biomass | 6.6-fold |
| Key Learning | GC content in the Shine-Dalgarno sequence significantly impacts RBS strength and final product titer. | - |
Enabling Technologies and Advanced Methodologies
Detailed Experimental Protocols
Protocol 1:In VitroPathway Prototyping with Crude Cell Lysates
Purpose: To rapidly test the functionality of the dopamine biosynthetic pathway and determine the optimal ratio of pathway enzymes (HpaBC to Ddc) before moving to in vivo engineering [24].
Procedure:
- Lysate Preparation: Grow the production E. coli host strain (e.g., FUS4.T2) to mid-log phase. Harvest cells by centrifugation and lyse them using a high-pressure homogenizer or sonication. Clarify the lysate by centrifugation to remove cell debris [24].
- Reaction Setup: Prepare a concentrated reaction buffer (e.g., 50 mM phosphate buffer, pH 7) containing essential co-factors: 0.2 mM FeCl₂, 50 µM vitamin B6 (PLP), and 1 mM L-tyrosine as the substrate [24].
- Pathway Assembly: Combine the reaction buffer with the crude cell lysate. Add plasmid DNA templates carrying the hpaBC and ddc genes under the control of inducible promoters.
- Incubation and Sampling: Incubate the reaction mixture at 30°C with shaking. Take samples at regular intervals (e.g., 0, 2, 4, 8 hours).
- Analysis: Quench the reactions and analyze the samples via High-Performance Liquid Chromatography (HPLC) to quantify the concentrations of L-tyrosine, L-DOPA, and dopamine. This data reveals the kinetics and efficiency of the pathway in vitro [24].
Protocol 2: High-Throughput RBS Engineering forIn VivoTuning
Purpose: To translate the optimal enzyme expression ratios identified in vitro into the production host by systematically varying the translation initiation rates of the hpaBC and ddc genes [24].
Procedure:
- RBS Library Design: Design a library of RBS sequences with varying Shine-Dalgarno (SD) sequences. Tools like the UTR Designer can be used, but a simplified approach focuses on modulating the SD sequence itself to alter strength without creating complex secondary structures [24].
- DNA Assembly: Use automated, high-throughput molecular cloning techniques (e.g., Golden Gate assembly) to construct the variant RBS sequences upstream of the hpaBC and ddc genes in the target expression vector [1].
- Transformation and Screening: Transform the library of constructs into the engineered E. coli production host. Plate on selective media and pick hundreds to thousands of colonies using an automated colony picker.
- High-Throughput Cultivation: Inoculate the picked colonies into deep-well plates containing a defined minimal medium. Use an automated fermentation system to grow the cultures under controlled conditions and induce gene expression [24].
- Product Quantification: After fermentation, lyse the cells and analyze the supernatant from each culture using HPLC or LC-MS to measure dopamine production. Correlate production titers with the specific RBS sequence for each strain [24].
The Role of Automation and Machine Learning
The implementation of knowledge-driven DBTL is greatly accelerated by biofoundries—automated laboratories that integrate robotics and software to execute build and test phases with high precision and throughput [24] [54]. For instance, the Illinois Biological Foundry for Advanced Biomanufacturing (iBioFAB) has demonstrated fully automated workflows for protein engineering, handling steps from mutagenesis PCR and DNA assembly to transformation, protein expression, and enzyme assays without human intervention [54].
Machine learning models are the intelligence that powers the "Learn" phase. In the LDBT paradigm, models like ESM-2 (a protein language model) and EVmutation (an epistasis model) can be used for zero-shot design of initial variant libraries with high diversity and quality [54]. As experimental data is generated, low-data machine learning models can be trained to predict variant fitness, guiding the selection of candidates for the next round of engineering and enabling a closed-loop, autonomous optimization process [2] [54].
The knowledge-driven DBTL cycle, exemplified by the successful engineering of a high-yield dopamine strain, represents a paradigm shift in biological design. By front-loading the process with mechanistic insights from in vitro systems, researchers can de-risk the traditionally costly and time-consuming in vivo strain engineering phase. The integration of cell-free systems for rapid prototyping, biofoundries for automated high-throughput experimentation, and machine learning for intelligent design and prediction creates a powerful, synergistic framework [24] [2] [54].
As these technologies continue to mature, the line between in vitro and in vivo development will further blur. The emergence of the LDBT cycle signals a future where predictive models and AI play an even more central role, potentially enabling "first-pass success" in many strain engineering projects. This advanced, knowledge-driven approach is set to dramatically accelerate the development of novel microbial cell factories for a more sustainable and healthier future, firmly establishing the DBTL cycle's critical role in the progression of biological engineering research.
Validating Success: Comparative Analysis of DBTL in Industrial and Clinical Settings
The Design-Build-Test-Learn (DBTL) cycle represents a foundational framework in modern biological engineering, enabling a systematic, iterative approach to strain development and bioprocess optimization. This engineering paradigm has transformed biosystems design from an artisanal, trial-and-error process into a disciplined, data-driven science. By implementing rapid, automated iterations of designing genetic constructs, building strains, testing their performance, and learning from the resulting data, research and development teams can achieve dramatic reductions in both development timelines and associated costs. The core power of the DBTL cycle lies in its iterative nature; each cycle generates quantitative data that informs and refines the subsequent design, progressively converging on an optimal solution with unprecedented speed [55] [44].
Within biofoundries—integrated facilities that combine robotic automation, advanced software, and synthetic biology—the DBTL cycle is executed at a scale and speed unattainable through manual methods. This acceleration is critical for addressing the inherent complexity of biological systems. As noted in analyses of biofoundry development, automating the DBTL cycle allows researchers to navigate vast biological design spaces efficiently, a task that would be otherwise prohibitively time-consuming and expensive [55]. For instance, what might take a graduate student weeks to accomplish manually can be reduced to days or even hours within an automated biofoundry environment, fundamentally altering the economics of biological R&D [55].
Quantitative Impact of DBTL Implementation
The implementation of automated DBTL cycles has yielded measurable, and often dramatic, improvements in R&D efficiency and cost-effectiveness. The table below summarizes key quantitative results from documented case studies across academia and industry.
Table 1: Quantitative Impacts of DBTL Cycles in Bioprocess and Strain Development
| Application Area | Reported Improvement | Impact Metric | Source/Context |
|---|---|---|---|
| Automated Strain Construction | 10-fold increase in throughput | 2,000 transformations/week (automated) vs. ~200/week (manual) | [56] |
| Dopamine Production in E. coli | 2.6 to 6.6-fold increase in performance | Titer: 69.03 mg/L (DBTL strain) vs. 27 mg/L (state-of-the-art) | [11] |
| Intensified Antibody Biomanufacturing | 37% reduction in process duration; 116% increase in product formation | Resulted in a 3-fold increase in space-time yield | [57] |
| Biofoundry Strain Testing | Significant reduction in cost per strain test | Cost lower than manual testing, as reported by Ginkgo Bioworks (2017) | [55] |
| Industrial Bioproduct Development | 20+ fold increase in productivity | Successfully commercialized 15 new substances in 7 years (Amyris) | [55] |
These case studies demonstrate that the DBTL framework delivers tangible value by shortening development timelines and enhancing product yields. The 10-fold improvement in the throughput of strain construction is a direct result of automating the "Build" phase, which eliminates a major bottleneck in metabolic engineering projects [56]. Furthermore, the ability to rapidly prototype and test genetic designs, as seen in the dopamine production case, allows for more efficient exploration of the genetic design space, leading to superior production strains in fewer iterations [11]. In industrial biomanufacturing, the application of DBTL principles to process intensification strategies leads to more productive and economically viable processes, as evidenced by the triple space-time yield for antibody production [57].
Detailed Experimental Protocols in a DBTL Workflow
Protocol 1: Automated High-Throughput Yeast Strain Construction
This protocol, adapted from an automated pipeline for screening biosynthetic pathways in yeast, details the "Build" phase of a DBTL cycle [56].
- Objective: To automate the transformation of Saccharomyces cerevisiae for high-throughput strain construction, achieving a throughput of ~2,000 transformations per week.
- Key Materials & Reagents:
- Competent Yeast Cells: Prepared in-house for high-efficiency transformation.
- Plasmid DNA: Library of expression plasmids containing genes of interest.
- Transformation Mix: Lithium acetate, single-stranded carrier DNA (ssDNA), and polyethylene glycol (PEG). Note: PEG viscosity requires optimized pipetting parameters on the robotic system.
- Selective Media: Solid and liquid media lacking specific nutrients for plasmid selection.
- Equipment: Hamilton Microlab VANTAGE robotic platform, integrated with an Inheco ODTC thermocycler (for heat shock), a plate sealer, and a plate peeler.
- Methodology:
- Transformation Setup and Heat Shock: The robotic system aliquots competent yeast cells into a 96-well plate, followed by the addition of plasmid DNA and the transformation mix (lithium acetate/ssDNA/PEG). The plate is sealed, mixed, and transferred by the robotic arm to the off-deck thermal cycler for a programmed heat shock.
- Washing: Following heat shock, the plate is centrifuged, the supernatant is removed, and cells are resuspended in a recovery medium.
- Plating: The cell suspension is transferred onto solid selective media in omni trays.
- Downstream Processing: Following incubation, transformed colonies are picked using an automated system (e.g., QPix 460) and inoculated into deep-well plates for high-throughput culturing in the "Test" phase.
- Critical Parameters: The protocol's success hinges on optimizing liquid handling classes for viscous reagents like PEG and integrating external hardware seamlessly to allow for hands-free operation after initial deck setup.
Protocol 2: Knowledge-Driven DBTL for Dopamine Production
This protocol exemplifies a "knowledge-driven" DBTL cycle, where upstream in vitro testing informs the in vivo strain engineering, making the "Design" phase more efficient [11].
- Objective: To develop and optimize an E. coli dopamine production strain by combining in vitro pathway testing with high-throughput in vivo RBS (Ribosome Binding Site) engineering.
- Key Materials & Reagents:
- Bacterial Strains: E. coli FUS4.T2, engineered for high L-tyrosine production, used as the production host.
- Plasmids: pJNTN plasmid system for hosting the dopamine biosynthetic pathway genes (hpaBC and ddc).
- Reaction Buffer for Cell Lysate Studies: Phosphate buffer (50 mM, pH 7) supplemented with FeCl₂, vitamin B6, and L-tyrosine or L-DOPA.
- Analytical Equipment: LC-MS system for quantifying dopamine titers.
- Methodology:
- In Vitro Investigation (Pre-DBTL): A crude cell lysate system is used to express the enzymes HpaBC and Ddc and test different relative expression levels. This upstream investigation provides mechanistic insight and identifies promising expression level ratios before moving to in vivo work.
- Design & Build: Based on the in vitro results, a library of genetic constructs is designed where the RBS sequences preceding hpaBC and ddc are systematically varied to fine-tune translation initiation rates. This library is built using high-throughput molecular cloning.
- Test: The constructed strains are cultured in a high-throughput format (e.g., in microtiter plates with selective media). A rapid, Zymolyase-mediated chemical extraction method is used to lyse cells, and dopamine titers are quantified using a streamlined LC-MS method (19-minute runtime).
- Learn: The performance data (dopamine titer for each RBS variant) is analyzed to identify optimal RBS combinations. This learning feeds directly into the next DBTL cycle for further refinement.
- Critical Parameters: The Shine-Dalgarno sequence's GC content is a critical factor influencing RBS strength and requires careful design. Using a high L-tyrosine production host is essential to ensure an ample precursor supply.
Essential Research Reagent Solutions
The successful execution of DBTL cycles relies on a suite of specialized reagents and tools. The following table catalogues key solutions used in the featured protocols and the broader field.
Table 2: Key Research Reagent Solutions for DBTL Workflows
| Reagent / Solution | Function in DBTL Cycle | Specific Application Example |
|---|---|---|
| Competent Cell Preparations | "Build": Essential for introducing engineered DNA into a host chassis. | High-efficiency S. cerevisiae or E. coli cells for transformation [56] [11]. |
| Standardized Genetic Parts (Promoters, RBS) | "Design": Modular, well-characterized DNA components for predictable system design. | RBS library for tuning gene expression in dopamine pathway [11]; inducible promoters (pLac, pTet) in biosensor design [43]. |
| Cell Lysis & Metabolite Extraction Kits | "Test": Enable high-throughput, rapid preparation of samples for analytics. | Zymolyase-mediated lysis for yeast [56]; organic solvent extraction for metabolites like verazine [56] and dopamine [11]. |
| Analytical Standards & Kits | "Test": Provide benchmarks for accurate identification and quantification of target molecules. | Used in LC-MS for verazine [56] and dopamine [11] quantification. |
| Specialized Culture Media | "Build"/"Test": Supports growth and production of engineered strains under selective pressure. | Selective media for plasmid maintenance; enriched basal media for fed-batch processes [57] [11]. |
Workflow Visualization and Logical Pathways
The Core DBTL Cycle in Biofoundries
The following diagram illustrates the continuous, automated flow of the DBTL cycle as implemented in a modern biofoundry, highlighting the integration of automation and data science at each stage.
Automated Strain Construction Workflow
This diagram details the specific unit operations and decision points within the "Build" phase of an automated DBTL cycle for yeast strain engineering, as described in the experimental protocol.
The quantitative data and detailed protocols presented in this guide unequivocally demonstrate that the disciplined application of the DBTL cycle, particularly when enhanced by automation and data science, is a powerful strategy for compressing R&D timelines and reducing development costs in biological engineering. The framework moves biological design from a slow, linear process to a fast, iterative one, enabling a more efficient and predictive path from concept to viable product. As the underlying technologies of automation, analytics, and machine learning continue to advance, the DBTL cycle is poised to become even more central to innovation across biomedicine, industrial biotechnology, and sustainable manufacturing.
The Design-Build-Test-Learn (DBTL) cycle is the cornerstone of modern biological engineering, driving the advancement of industrial biomanufacturing from traditional antibiotic production to the creation of novel bio-based materials. This iterative framework accelerates the development of microbial cell factories by systematically designing genetic constructs, building them into host organisms, testing the resulting phenotypes, and learning from data to inform the next design cycle. The integration of advanced tools like artificial intelligence (AI), CRISPR gene editing, and synthetic biology into the DBTL cycle has dramatically increased the speed and efficiency of bioprocess development, enabling a shift toward a more sustainable and circular bioeconomy. This whitepaper explores current trends, quantitative market data, and detailed experimental methodologies that define the modern industrial biotechnology landscape, providing researchers and scientists with a technical guide to navigating this rapidly evolving field.
Quantitative Market Landscape and Core Technologies
The industrial biotechnology market is experiencing significant growth, fueled by technological advancements and a global push for sustainability. The table below summarizes key market data and growth projections.
Table 1: Industrial Biotechnology Market Overview and Growth Drivers
| Metric | 2024/2025 Value | 2034 Projection | Key Growth Drivers |
|---|---|---|---|
| Global Biotech Market [58] | USD 1.744 trillion (2025) | USD 5+ trillion | Accelerated growth from AI, advanced therapies, and bioconvergence. |
| Industrial Biotech Market [59] | USD 585.1 million (2024) | USD 1.47 billion | CAGR of 9.63% (2025-2034); demand for sustainable, bio-based alternatives. |
| AI Impact on Preclinical Discovery [60] | - | 30-50% shorter timelines, 25-50% lower costs | AI-driven compound screening and design. |
| Top R&D Priorities [61] | Oncology (64%), Immunology (41%), Rare Diseases (31%) | - | Focus on high-ROI therapeutic areas with significant unmet need. |
Enabling Technologies Reshaping the DBTL Cycle
- AI and Machine Learning: AI technologies are revolutionizing the "Design" and "Learn" phases. Machine learning algorithms analyze genomic and proteomic data to predict optimal microbial strains, optimize fermentation processes, and decrease experimental timescales [59]. AI-driven platforms have reported Phase 1 success rates greater than 85% in some cases, with the potential to reduce preclinical discovery time by 30-50% [60].
- CRISPR and Advanced Gene Editing: CRISPR-based gene editing is a pivotal tool in the "Build" phase, enabling precise engineering of microorganisms for specific applications. It allows for the creation of high-performing microbial strains, improvement of enzyme performance, and development of organisms resistant to process-specific stressors [59].
- Bioconvergence: The convergence of biology, engineering, and computing is reaching mainstream adoption. Applications span organ-on-a-chip diagnostics, sustainable bio-based materials, and cultivated foods. The Asia Pacific segment of this market is growing rapidly and is expected to reach USD 60.7 billion by 2030 [58].
- Sustainable Production Models: There is a rising demand for bio-based products and a shift toward a circular economy. Industrial biotechnology enables the production of biofuels, biodegradable plastics, and biochemicals from renewable resources, reducing dependence on fossil fuels and decreasing carbon emissions [59].
Experimental Protocols in Industrial Biomanufacturing
This section provides detailed methodologies for key biomanufacturing processes, illustrating the practical application of the DBTL cycle.
Protocol 1: Microbial Biosynthesis of Polyhydroxyalkanoates (PHA)
Objective: To engineer microbial strains for the high-yield production of PHA biopolymers from carbon-rich feedstocks.
Table 2: Key Research Reagents for PHA Biosynthesis
| Research Reagent | Function in the Experiment |
|---|---|
| Cupriavidus necator (e.g., ATCC 17699) | Model gram-negative bacterium used as a microbial chassis for PHA biosynthesis [62]. |
| Recombinant E. coli (engineered) | Common host for recombinant PHA pathways, often modified with genes from Aeromonas caviae or Ralstonia eutropha [62]. |
| Waste Glycerol or C1 Gases (e.g., CO₂, Methane) | Low-cost, sustainable carbon source for PHA production [62]. |
| Propionic Acid | Co-substrate fed during fermentation to promote the synthesis of P(3HB-co-3HV) copolymers [62]. |
| Chloroform | Primary solvent used for the extraction and purification of PHA from microbial biomass [62]. |
| Methanol | Used to wash and precipitate PHA polymers post-extraction to remove residual cell debris and solvents [62]. |
Detailed Methodology:
- Strain Engineering (Build):
- For Cupriavidus necator, introduce genes for the synthesis of specific PHA copolymers (e.g., P(3HB-co-3HV)) via plasmid transformation or chromosomal integration.
- For recombinant production in E. coli, express the phbA (β-ketothiolase), phbB (acetoacetyl-CoA reductase), and phbC (PHA synthase) operon from a suitable vector [62].
- Preculture and Fermentation (Test):
- Inoculate a single colony into a rich medium (e.g., LB) and incubate overnight at 30-37°C with shaking.
- Transfer the preculture to a bioreactor containing a defined mineral medium with the primary carbon source (e.g., 20 g/L waste glycerol). For copolymer production, feed propionic acid (e.g., 1-2 g/L) in a controlled manner to avoid toxicity.
- Maintain fermentation parameters: pH at 6.8-7.2, temperature at 30-37°C, dissolved oxygen above 20% saturation. Allow the fermentation to proceed for 48-72 hours [62].
- PHA Extraction and Analysis (Test):
- Harvest cells by centrifugation (e.g., 8,000 x g, 10 min).
- Lyophilize the cell pellet and weigh it to determine dry cell weight.
- For extraction, suspend the biomass in chloroform (1:10 w/v) and incubate at 60°C for 2-4 hours with occasional mixing.
- Filter the chloroform solution to remove cell debris, then concentrate it via rotary evaporation.
- Precipitate the polymer by adding the concentrated solution to a 10-fold volume of cold methanol. Recover the purified PHA by filtration and dry it under vacuum [62].
- Characterization (Learn):
- Determine PHA content gravimetrically.
- Analyze polymer composition using Gas Chromatography-Mass Spectrometry (GC-MS) after methanolysis of the polymer to its constituent hydroxy acid methyl esters.
- Characterize molecular weight and distribution via Gel Permeation Chromatography (GPC).
Protocol 2: Engineering a Synthetic Carbon Fixation Pathway in Plants
Objective: To design and implement an artificial carbon fixation cycle (the Malyl-CoA glycerate cycle) in a model plant to enhance growth and lipid production.
Detailed Methodology:
- Pathway Design and Vector Construction (Design & Build):
- Design: Model the synthetic McG cycle to interface with the native Calvin–Benson–Bassham (CBB) cycle, ensuring metabolite compatibility and reduced photorespiration [63].
- Build: Clone the necessary bacterial or synthetic genes encoding the key enzymes of the McG cycle (e.g., malyl-CoA synthetase, malyl-CoA lyase) into a plant expression vector. Use strong, constitutive plant promoters (e.g., CaMV 35S) and target the proteins to the chloroplast using appropriate transit peptides [63].
- Plant Transformation and Selection (Build):
- Use Agrobacterium tumefaciens-mediated transformation for the model plant Arabidopsis thaliana.
- Sterilize and germinate seeds on selective media containing antibiotics (e.g., kanamycin) to identify positive transformants.
- Select transgenic lines (T1 generation) and grow to maturity to produce T2 seeds [63].
- Phenotypic Screening and Analysis (Test):
- Growth Phenotype: Grow T2 transgenic and wild-type plants under controlled conditions. Measure rosette diameter, plant height, and fresh/dry biomass after 4-6 weeks. Successful transformants may show a 2-3 times increase in biomass [63].
- Lipid Analysis: Harvest plant tissues and perform lipid extraction using a chloroform-methanol mixture. Quantify total lipids gravimetrically and analyze fatty acid profiles via GC-MS [63].
- Metabolite Profiling: Use GC-MS or LC-MS to analyze intermediate metabolites of both the CBB and McG cycles to confirm the operation of the synthetic pathway [63].
- Validation and Further Engineering (Learn):
- Analyze data to confirm enhanced carbon fixation efficiency (reported up to 50% increase) and correlate with growth and lipid production phenotypes [63].
- Use these findings to inform the next DBTL cycle, potentially optimizing gene expression levels or transferring the system to economically important crops like rice or tomatoes [63].
Visualizing the DBTL Cycle and Metabolic Pathways
The following diagrams, created with Graphviz, illustrate the core DBTL framework and a key metabolic pathway described in the protocols.
The DBTL Cycle in Biological Engineering
Engineered PHA Biosynthesis Pathway
Challenges and Future Outlook
Despite its promise, the field faces several significant challenges. Regulatory complexities surrounding genetically modified organisms (GMOs) can lead to prolonged approval timelines and increased compliance costs [59]. Funding gaps, particularly for early-stage research and small biotechs, pose a major hurdle, with government funding cuts further exacerbating the situation [58]. The high cost of R&D and infrastructure remains a barrier, as scaling up from lab-scale to industrial production is capital-intensive and fraught with technical risks [59]. Finally, a shortage of skilled talent in areas spanning AI, engineering, and regulatory science constrains the pace of innovation [58].
The future of industrial biomanufacturing is intrinsically linked to the continued refinement and acceleration of the DBTL cycle. Key trends point toward an increased reliance on AI for predictive biology, the expansion of biomanufacturing into the production of a wider array of complex materials and chemicals, and a stronger focus on circular economy principles where waste streams become feedstocks. As bioconvergence deepens, the integration of biology with advanced engineering and computing will undoubtedly unlock new capabilities, solidifying industrial biotechnology's role as a cornerstone of a sustainable future.
The development of cell and gene therapies (CGTs) represents a frontier in modern medicine, offering potential cures for conditions with limited therapeutic options. However, the transition from laboratory research to clinically approved treatments remains fraught with challenges. Current data reveals that CGT products have an overall likelihood of approval (LOA) of just 5.3%, with variability based on therapeutic area—oncology indications show a lower LOA (3.2%) compared to non-oncology indications (8.0%) [64]. The high failure rates stem from multiple factors, including complex biology, manufacturing challenges, and the limitations of conventional development paradigms.
The conventional linear approach to therapy development—progressing sequentially from preclinical studies through Phase 1, 2, and 3 trials—often proves inefficient for CGTs. These therapies frequently exhibit complex mechanism-of-action relationships where traditional surrogate endpoints may not reliably predict long-term clinical benefit [65]. A documented trial in multiple myeloma exemplifies this challenge, where an interim analysis based on early response data suggested futility, while subsequent analysis of progression-free survival demonstrated clear therapeutic benefit [65]. This disconnect between early biomarkers and long-term outcomes underscores the need for more integrated development approaches.
The Design-Build-Test-Learn (DBTL) cycle, a cornerstone of synthetic biology, offers a transformative framework for addressing these challenges. This iterative engineering paradigm enables continuous refinement of therapeutic designs based on empirical data, potentially accelerating the optimization of CGT products. Recent advances have even proposed a reformulation to LDBT (Learn-Design-Build-Test), where machine learning on existing biological data precedes design, potentially reducing the need for multiple iterative cycles [3] [2]. This review explores how these engineered approaches, combined with innovative clinical trial designs and analytical tools, are reshaping the clinical translation of cell and gene therapies.
The DBTL Cycle: An Engine for Preclinical Optimization
Core Principles and Workflow
The DBTL cycle provides a systematic framework for engineering biological systems, with each phase contributing distinct activities toward therapeutic optimization:
- Design: In this initial phase, researchers define target product profiles and specify genetic constructs, cellular systems, or vector designs. For chimeric antigen receptor (CAR) T-cell therapies, this includes selecting target antigens, signaling domains, and gene expression elements.
- Build: This phase involves the physical construction of genetic elements and their introduction into cellular hosts. Advanced tools such as high-throughput DNA synthesis, CRISPR-based editing, and automated cloning systems enable rapid assembly of therapeutic candidates.
- Test: Constructed therapies undergo rigorous functional characterization using in vitro and in vivo models. Cell-free expression systems have emerged as particularly valuable tools, enabling rapid protein characterization—often within hours rather than days required for living host cells [3].
- Learn: Data from testing phases are analyzed to refine understanding of structure-function relationships and identify optimization opportunities. Machine learning algorithms can detect complex patterns in high-dimensional data, generating predictive models that inform the next design cycle [2].
The integration of computational tools across this cycle enables more predictive design, significantly accelerating the optimization process. For protein engineering, sequence-based language models (ESM, ProGen) and structure-based tools (ProteinMPNN, AlphaFold) facilitate zero-shot predictions of protein functionality without additional experimental training data [2].
Table 1: Key Computational Tools for CGT Development
| Tool Category | Representative Examples | Applications in CGT Development |
|---|---|---|
| Protein Language Models | ESM, ProGen | Predicting beneficial mutations, inferring protein function [2] |
| Structure-Based Design | ProteinMPNN, MutCompute | Designing protein variants with enhanced stability and activity [2] |
| Functional Prediction | Prethermut, Stability Oracle, DeepSol | Optimizing protein thermostability and solubility [2] |
| Biosensor Design | T-SenSER computational platform | Creating synthetic receptors with programmable signaling [66] |
Case Study: Engineering Programmable Biosensors
The development of TME-sensing switch receptors for enhanced response to tumors (T-SenSER) exemplifies the power of computational design in CGT development. Researchers created a computational protein design platform for de novo assembly of allosteric receptors that respond to soluble tumor microenvironment (TME) factors [66]. The platform involved:
- Selecting structural elements defining input (ligand-binding domains) and output (signaling domains)
- Assembling multi-domain scaffolds using RoseTTAFold and AlphaFold2
- Ranking receptor scaffolds based on dimerization propensity and long-range communication between domains
The resulting T-SenSERs, targeting VEGF or CSF1 in the TME, demonstrated enhanced anti-tumor responses when combined with conventional CAR-T cells in models of lung cancer and multiple myeloma [66]. This approach enables the creation of synthetic biosensors with custom-built sensing and response properties, potentially overcoming the immunosuppressive signals that often limit CGT efficacy in solid tumors.
Innovative Clinical Development Strategies
Novel Clinical Trial Designs
Traditional clinical trial designs often struggle to efficiently evaluate the complex risk-benefit profiles of CGTs. Innovative designs that integrate earlier phases of development offer promising alternatives:
Gen 1-2-3 Design: This generalized phase 1-2-3 design begins with phase 1-2 dose-finding but identifies a set of candidate doses rather than a single dose [65]. In stage 2, patients are randomized among candidate doses and an active control, with survival time data used to select an optimal dose. A Go/No Go decision for phase 3 is based on the predictive probability that the selected dose will provide substantive improvement over control [65].
PKBOIN-12 Design: This Bayesian optimal interval design incorporates pharmacokinetic outcomes alongside toxicity and efficacy data for optimal biological dose selection [67]. By leveraging PK data (e.g., AUC, Cmax) that become available much faster than clinical outcomes, the design enhances OBD identification and improves patient allocation to efficacious doses.
Table 2: Quantitative Success Rates in Cell and Gene Therapy Development [64]
| Development Stage | Probability of Phase Success | Factors Influencing Success |
|---|---|---|
| Phase I to Phase II | 60.2% | Orphan designation, therapeutic area |
| Phase II to Phase III | 42.9% | Strong proof-of-concept data |
| Phase III to Submission | 66.7% | Robust trial design, endpoint selection |
| Submission to Approval | 87.1% | Complete evidence package, manufacturing quality |
| Overall Likelihood of Approval | 5.3% | Orphan: 9.4%, Non-orphan: 3.2%, Oncology: 3.2%, Non-oncology: 8.0% |
Integrated Evidence Generation
Successful CGT development requires strategic evidence generation planning across the product lifecycle:
Natural History Studies: These studies provide critical information on disease progression and inform clinical outcome assessments. Genetic testing within natural history studies can identify patient subpopulations more likely to benefit from therapy [68].
Patient-Centric Protocols: Decentralized study elements and virtual research coordinating centers can enhance patient recruitment and retention while minimizing burden—particularly important for long-term follow-up requirements of 5-15 years [68].
Real-World Evidence (RWE): Strategically collected RWE can support regulatory submissions and fulfill post-approval requirements, providing insights into therapy performance in broader patient populations [68].
The Scientist's Toolkit: Essential Research Reagents and Platforms
Table 3: Essential Research Reagents and Platforms for CGT Development
| Reagent/Platform | Function/Application | Key Features |
|---|---|---|
| Cell-Free Transcription-Translation (TX-TL) Systems | Rapid testing of genetic constructs [3] | Bypasses complexities of living cells; enables testing within hours; high-throughput capability |
| Synthetic Interfaces (SpyTag/SpyCatcher, Coiled-Coils) | Modular enzyme assembly for natural product biosynthesis [69] | Standardized connectors; orthogonal functionality; post-translational complex formation |
| Computational Protein Design Platforms | De novo receptor design [66] | Customizable input-output behaviors; structure prediction (AlphaFold2, RoseTTAfold) |
| Droplet Microfluidics | High-throughput screening [2] | Enables screening of >100,000 reactions; picoliter-scale reactions; reduced reagent costs |
| CAR Signaling Domains | T-cell activation and persistence [66] | Co-stimulatory domains (CD28, 4-1BB); cytokine signaling domains (c-MPL) |
Integrated Workflows: From DBTL to Clinical Translation
The most successful CGT development programs integrate preclinical and clinical activities through coordinated workflows. The diagram below illustrates how the DBTL cycle informs clinical development, creating a continuous feedback loop that accelerates optimization.
The integration of machine learning and rapid testing platforms enables a shift toward LDBT (Learn-Design-Build-Test) approaches, where learning from existing datasets precedes design [3]. This paradigm can generate functional parts and circuits in a single cycle, moving synthetic biology closer to a "Design-Build-Work" model similar to established engineering disciplines [2].
For clinical development, innovative designs like the Gen 1-2-3 design create decision points that incorporate longer-term outcome measures rather than relying solely on early surrogates [65]. The incorporation of pharmacokinetic data in designs like PKBOIN-12 provides earlier insights into exposure-response relationships [67].
The clinical translation of cell and gene therapies remains challenging but is being accelerated through the systematic application of engineering principles. The DBTL cycle, enhanced by machine learning and high-throughput testing technologies, provides a powerful framework for optimizing therapeutic candidates before they enter clinical development. Integrated clinical trial designs that incorporate dose optimization and Go/No Go decisions based on predictive probabilities offer more efficient pathways to demonstrating efficacy and safety.
As these approaches mature, the development of CGTs will likely become more predictable and efficient. The convergence of computational design, rapid testing platforms, and innovative clinical methodologies holds the promise of delivering transformative therapies to patients more rapidly while managing the inherent risks of development. Success in this endeavor requires multidisciplinary collaboration across computational biology, synthetic biology, and clinical development—a integration that represents the future of therapeutic innovation.
The Design-Build-Test-Learn (DBTL) cycle represents a foundational framework in modern biological engineering, offering a structured, iterative alternative to traditional trial-and-error approaches. This systematic methodology has transformed strain and bioprocess development by integrating computational design, automated construction, high-throughput testing, and data-driven learning [1] [11]. Within synthetic biology, DBTL enables the rational reprogramming of organisms through engineering principles, moving beyond the limitations of ad-hoc experimentation [20]. This whitepaper provides a comparative analysis of DBTL versus traditional methods, examining their respective impacts on project timelines, outcomes, and overall efficiency within biological engineering research and drug development.
The traditional trial-and-error approach often relies heavily on researcher intuition, sequential experimentation, and manual techniques. This process can be slow, resource-intensive, and difficult to scale, particularly when optimizing complex biological systems with numerous interacting variables [1] [11]. In contrast, the DBTL framework establishes a standardized operational mode for industrial biotechnology that addresses the lengthy and costly traditional trial-and-error process by combining manual and robotic protocols with specialized software [70].
Fundamental Methodological Differences
The DBTL Cycle Framework
The DBTL cycle operates as an integrated, iterative system with four distinct phases:
Design: This initial phase applies rational principles to design biological components and systems. Leveraging modular design of DNA parts enables assembly of diverse construct varieties by interchanging individual components [1]. Computational tools and models support this phase, with recent advancements incorporating machine learning to process large biological datasets [20].
Build: This phase involves physical construction of biological systems, typically through DNA assembly and molecular cloning. Automation has revolutionized this stage, significantly reducing the time, labor, and cost of generating multiple constructs while increasing throughput [1] [71]. Advanced genetic engineering tools and biofoundries enable high-throughput automated assembly [20].
Test: Constructs are analyzed through functional assays to evaluate performance. Automation and high-throughput screening methods have dramatically increased testing capacity, allowing characterization of thousands of variants [71]. Biofoundries leverage next-generation sequencing and mass spectrometry to collect large amounts of multi-omics data at single-cell resolution [20].
Learn: Data from testing phases are analyzed to inform subsequent design cycles. This phase employs statistical evaluation and model-guided assessment, with machine learning techniques increasingly used to refine biological system performance [11] [20]. The learning phase closes the loop, transforming raw data into actionable knowledge for improved designs.
Traditional Trial-and-Error Approach
Traditional methods in biological engineering typically involve:
- Sequential Experimentation: Linear hypothesis testing without systematic iteration cycles
- Researcher-Dependent Intuition: Heavy reliance on individual expertise and prior experience
- Limited Parallelization: Manual techniques restricting throughput and scalability
- Minimal Data Integration: Isolated experiments without comprehensive data synthesis
- Empirical Optimization: Parameter adjustment based on observable outcomes rather than predictive modeling
Quantitative Comparative Analysis
Performance Metrics Comparison
Table 1: Direct comparison of key performance metrics between DBTL and traditional methods
| Performance Metric | DBTL Approach | Traditional Trial-and-Error |
|---|---|---|
| Experiment Throughput | High (100s-1000s of variants) [20] | Low (limited by manual capacity) |
| Iteration Cycle Time | Weeks to months [11] | Months to years |
| Resource Requirements | High initial investment, lower per-data point | Consistently high throughout project |
| Data Generation Capacity | Massive multi-omics datasets [20] | Limited by experimental design |
| Success Rate Optimization | 2.6 to 6.6-fold improvement demonstrated [11] | Incremental improvements |
| Automation Compatibility | Fully compatible with biofoundries [70] | Limited compatibility |
| Predictive Modeling Support | Strong (ML and computational models) [20] | Minimal |
Case Study: Dopamine Production Optimization
A recent study developing an optimized dopamine production strain in Escherichia coli provides compelling quantitative evidence of DBTL effectiveness [11]. The knowledge-driven DBTL cycle, incorporating upstream in vitro investigation, enabled both mechanistic understanding and efficient cycling.
Table 2: Dopamine production optimization results using DBTL cycle [11]
| Methodological Approach | Dopamine Production | Improvement Factor | Key Features |
|---|---|---|---|
| State-of-the-Art Traditional | 27 mg/L | Baseline | Conventional strain engineering |
| DBTL Cycle Implementation | 69.03 ± 1.2 mg/L | 2.6-fold | High-throughput RBS engineering |
| DBTL with Host Engineering | 34.34 ± 0.59 mg/gbiomass | 6.6-fold | l-tyrosine overproduction host |
The DBTL implementation achieved this significant improvement through a systematic workflow: (1) in vitro cell lysate studies to investigate enzyme expression levels, (2) translation to in vivo environment through high-throughput ribosome binding site (RBS) engineering, and (3) development of a high l-tyrosine production host strain [11]. This approach combined mechanistic investigation with automated workflow execution, demonstrating how DBTL accelerates optimization while generating fundamental biological insights.
Experimental Protocols
DBTL Workflow for Metabolic Engineering
The knowledge-driven DBTL cycle for dopamine production exemplifies a robust protocol for metabolic engineering applications [11]:
Phase 1: Design
- Host Strain Selection: Choose production host (e.g., E. coli FUS4.T2) with precursor optimization (l-tyrosine overproduction)
- Pathway Design: Identify heterologous genes (hpaBC for l-DOPA synthesis, ddc for dopamine production)
- Modular Component Design: Plan interchangeable genetic parts for combinatorial assembly
Phase 2: Build
- DNA Assembly: Construct plasmids using high-throughput techniques (e.g., Gibson assembly)
- Library Generation: Create RBS variant libraries for expression tuning
- Strain Transformation: Introduce construct libraries into production host
Phase 3: Test
- High-Throughput Screening: Cultivate variants in 96-well plates with minimal medium
- Analytical Quantification: Measure dopamine production via HPLC or LC-MS
- Multi-Omics Data Collection: Extract transcriptomic, proteomic, and metabolomic data
Phase 4: Learn
- Data Integration: Correlate production titers with RBS sequences and expression levels
- Model Refinement: Update predictive models of pathway performance
- Design Optimization: Identify optimal construct combinations for next cycle
Traditional Metabolic Engineering Protocol
For comparative purposes, traditional metabolic engineering typically follows this sequence:
- Literature Review and Hypothesis Generation: Based on known pathways and mechanisms
- Single Construct Development: Sequential testing of individual genetic designs
- Manual Cloning and Transformation: Low-throughput molecular biology techniques
- Bench-Scale Fermentation: Time-consuming shake flask or bioreactor studies
- End-Point Analysis: Focus on final titer rather than comprehensive data collection
- Researcher Interpretation: Results evaluation based on experience and intuition
Visualization of Workflows
DBTL Cycle Workflow
Traditional Trial-and-Error Workflow
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential research reagents and tools for DBTL implementation
| Tool Category | Specific Examples | Function in DBTL Cycle |
|---|---|---|
| DNA Assembly Systems | Gibson assembly [20], Golden Gate cloning | Modular construction of genetic variants |
| Expression Vectors | pET system, pJNTN [11] | Genetic context for heterologous expression |
| Host Strains | E. coli FUS4.T2 [11], E. coli DH5α | Chassis for pathway implementation |
| Analytical Tools | HPLC, LC-MS, NGS [20] [11] | Quantification of products and system characterization |
| Automation Platforms | Liquid handlers, colony pickers [71] | High-throughput execution of build and test phases |
| Data Analysis Software | Galaxy platform [70], machine learning algorithms [20] | Learning phase implementation and model building |
| Cell-Free Systems | CFPS systems [11] | Rapid in vitro testing of pathway components |
Impact on Biological Engineering Research
Accelerating Discovery and Optimization
The implementation of DBTL cycles has dramatically accelerated biological engineering timelines. Where traditional methods might require years to optimize a single metabolic pathway, DBTL approaches can achieve comparable or superior results in months [11]. This acceleration stems from several factors: parallel testing of multiple variants, reduced manual intervention through automation, and data-driven decision making that minimizes unproductive experimental directions.
Biofoundries—facilities specializing in automated DBTL implementation—have emerged as critical infrastructure for modern biological engineering. The Global Biofoundry Alliance, established in 2019, coordinates these facilities worldwide to standardize approaches and share best practices [20]. These centers enable researchers to explore design spaces of unprecedented size, testing thousands of genetic variants rather than the handfuls feasible with manual methods.
Enhancing Fundamental Understanding
Beyond practical optimization, DBTL cycles generate comprehensive datasets that advance fundamental biological knowledge. The dopamine production case study [11] not only achieved higher titers but also revealed mechanistic insights about RBS strength and GC content in the Shine-Dalgarno sequence. This dual benefit—practical optimization coupled with basic science advancement—represents a significant advantage over traditional methods, which often prioritize immediate results over mechanistic understanding.
Machine learning integration represents the cutting edge of DBTL advancement. As noted in recent synthetic biology literature, ML can process big data and provide predictive models by choosing appropriate features and uncovering unseen patterns [20]. This capability addresses the "learn" bottleneck that has traditionally limited DBTL effectiveness, potentially enabling predictive biological design with reduced experimental iteration.
The comparative analysis clearly demonstrates the superiority of DBTL frameworks over traditional trial-and-error methods across multiple dimensions: accelerated project timelines, improved success rates, enhanced resource efficiency, and greater fundamental insight generation. Quantitative evidence from metabolic engineering case studies shows 2.6 to 6.6-fold improvements in key performance metrics when implementing knowledge-driven DBTL cycles [11].
For biological engineering researchers and drug development professionals, DBTL implementation requires significant upfront investment in infrastructure, computational resources, and interdisciplinary expertise. However, the long-term benefits—including faster development cycles, higher success probabilities, and more robust biological systems—justify this investment, particularly for organizations pursuing complex engineering challenges. As automation, machine learning, and data science continue advancing, the performance gap between DBTL and traditional approaches will likely widen further, establishing DBTL as the unequivocal standard for biological engineering research.
Conclusion
The DBTL cycle has firmly established itself as the central paradigm for rational biological engineering, transforming the field from an artisanal practice into a disciplined, data-driven science. The integration of AI for predictive design and automation for high-throughput execution is decisively overcoming the traditional 'learn' bottleneck, enabling a shift from iterative guessing to precise, knowledge-driven design. As evidenced by its success in creating efficient cell factories and accelerating advanced therapeutics like CAR-T cells, the DBTL framework is pivotal for advancing biomedical research. Future directions point toward fully autonomous, self-optimizing biological systems, explainable AI for deeper mechanistic insights, and the continued convergence of digital and biological technologies. This progression will undoubtedly unlock new frontiers in predictive cell biodesign, paving the way for personalized medicines and sustainable biomanufacturing solutions that are currently beyond our reach.
References
- 1. DBTL Approach in Synthetic Biology [https://www.moleculardevices.com/applications/synthetic-biology/dbtl-approach]
- 2. combining machine learning and rapid cell-free testing [https://www.nature.com/articles/s41467-025-65281-2]
- 3. LDBT: Machine Learning Meets Rapid Cell-Free Testing [https://bioengineer.org/ldbt-machine-learning-meets-rapid-cell-free-testing/]
- 4. Breaking the Bottleneck in AI-Driven Protein Engineering [https://www.ailurus.bio/post/breaking-the-bottleneck-in-ai-driven-protein-engineering]
- 5. Inside ELRIG's Drug Discovery 2025 [https://www.drugtargetreview.com/article/190237/inside-elrigs-drug-discovery-2025/]
- 6. Synthetic DNA Library Synthesis Service [https://synbio-tech.com/synthetic-DNA-library-synthesis-service]
- 7. The automated Galaxy-SynBioCAD pipeline for synthetic ... [https://www.nature.com/articles/s41467-022-32661-x]
- 8. Machine Learning and Deep Learning in Synthetic Biology [https://pmc.ncbi.nlm.nih.gov/articles/PMC10918679/]
- 9. Variant Libraries [https://www.twistbioscience.com/products/libraries]
- 10. Simulated Design–Build–Test–Learn Cycles for Consistent ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10510747/]
- 11. The knowledge driven DBTL cycle provides mechanistic ... [https://microbialcellfactories.biomedcentral.com/articles/10.1186/s12934-025-02729-6]
- 12. RNA Toehold Switch Optimization - NeuroSplice [https://2025.igem.wiki/calucsf/results]
- 13. DNA Libraries - GENEWIZ from Azenta Life ... [https://www.genewiz.com/public/services/gene-synthesis/synthetic-dna-libraries]
- 14. Engineering | ESSENTIAL KOREA - iGEM 2025 [https://2025.igem.wiki/essential-korea/engineering]
- 15. An automated Design-Build-Test-Learn pipeline for ... [https://www.nature.com/articles/s42003-018-0076-9]
- 16. Big Data in the Pharma Industry: Opportunities and ... [https://kms-technology.com/blog/big-data-in-pharma-industry/]
- 17. Data-driven synthetic microbes for sustainable future [https://www.nature.com/articles/s41540-025-00556-4]
- 18. Opportunities and Challenges of Using Big Data to Detect ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6617974/]
- 19. HTS Robotics Platform - The Wertheim UF Scripps Institute [https://hts.scripps.ufl.edu/facilities/hts-robotics/]
- 20. Learning the way toward high-precision biological design [https://pmc.ncbi.nlm.nih.gov/articles/PMC10231942/]
- 21. Automating the Future of Genetic Engineering [https://chenected.aiche.org/2025/04/automating-future-genetic-engineering]
- 22. High Throughput Screening [https://highresbio.com/applications/ready-work-cells/high-throughput-automation]
- 23. iAutoEvoLab as an all-in-one laboratory for programmable ... [https://www.nature.com/articles/s44286-025-00305-8]
- 24. The knowledge driven DBTL cycle provides mechanistic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12084978/]
- 25. The knowledge driven DBTL cycle provides mechanistic ... [https://pubmed.ncbi.nlm.nih.gov/40380156/]
- 26. 综述:生物铸造厂前沿:机遇与挑战 [https://www.ebiotrade.com/newsf/2025-10/20251006103932830.htm]
- 27. Complexity of dopamine metabolism - PMC - NIH [https://pmc.ncbi.nlm.nih.gov/articles/PMC3693914/]
- 28. Aromatic L-amino acid decarboxylase [https://en.wikipedia.org/wiki/Aromatic_L-amino_acid_decarboxylase]
- 29. hydroxylase and regulation of Tyrosine synthesis dopamine [https://pubmed.ncbi.nlm.nih.gov/21176768/]
- 30. Bacterial species-structure-property relationships of ... [https://microbialcellfactories.biomedcentral.com/articles/10.1186/s12934-025-02833-7]
- 31. 他深耕30多年,建立我国自己的万吨级PHA产线 [https://www.tsinghua.edu.cn/info/1182/118300.htm]
- 32. 课题组最新进展| 酵母也疯狂:小小细胞工厂竟能“发酵”出生物 ... [https://wap.sciencenet.cn/blog-3496796-1502070.html?mobile=1]
- 33. AI-informed approach to CAR design enhances bi-specific ... [https://www.stjude.org/media-resources/news-releases/2025-medicine-science-news/ai-informed-approach-to-car-design-enhances-bi-specific-car-t-cells.html]
- 34. Tracing the development of CAR-T cell design [https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1615212/full]
- 35. AI-Designed Proteins Can Boost Production of T Cells [https://hms.harvard.edu/news/ai-designed-proteins-can-boost-production-t-cells]
- 36. A Guide to Multi-omics Integration Strategies [https://frontlinegenomics.com/a-guide-to-multi-omics-integration-strategies/]
- 37. Generative AI in drug discovery and development [https://pmc.ncbi.nlm.nih.gov/articles/PMC11444559/]
- 38. Transformative Role of Artificial Intelligence in Drug ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12406033/]
- 39. Unleashing the power of generative AI in drug discovery [https://www.sciencedirect.com/science/article/pii/S135964462400117X]
- 40. Machine Learning in Drug Discovery Market 2025 Trends [https://www.towardshealthcare.com/insights/machine-learning-in-drug-discovery-market-sizing]
- 41. how generative AI can tackle undruggable targets [https://www.drugtargetreview.com/article/163308/next-generation-drug-design-ai-tackle-undruggable-targets/]
- 42. The Predictive Pipeline: Structuring Drug Development ... [https://www.drugpatentwatch.com/blog/the-predictive-pipeline-structuring-drug-development-timelines-with-ai-driven-patent-intelligence/?srsltid=AfmBOopw1p3-q-dq212pxIPAoYi5KKoU2Q2myMnmoq9MZdMivlZWOBqc]
- 43. Engineering - Iterative DBTL Cycles | LYON - iGEM 2025 [https://2025.igem.wiki/lyon/engineering]
- 44. Frontiers in biofoundry: opportunities and challenges [https://www.frontiersin.org/journals/synthetic-biology/articles/10.3389/fsybi.2025.1630026/full]
- 45. Architectures of emerging biofoundry platforms for synthetic ... [https://www.sciencedirect.com/science/article/abs/pii/S0958166925001235]
- 46. About [https://kbiofoundry.org/01_about.html]
- 47. Abstraction hierarchy to define biofoundry workflows and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12214803/]
- 48. Abstraction hierarchy to define biofoundry workflows and ... [https://www.nature.com/articles/s41467-025-61263-6]
- 49. Biofoundry-as-a-Service Market Research Report 2033 [https://dataintelo.com/report/biofoundry-as-a-service-market]
- 50. How Biofoundries are Accelerating Life-Enhancing ... [https://www.synbiobeta.com/read/how-biofoundries-are-accelerating-life-enhancing-innovations-in-biomanufacturing]
- 51. Sensors for Smart : Enhancing Industrial Efficiency... Process Control [https://www.iaasiaonline.com/smart-sensors-for-process-control-enhancing-industrial-efficiency-with-real-time-data/]
- 52. Sensors in industrial automation | Adaptive Smart Systems Control [https://www.plantautomation-technology.com/articles/smart-sensors-revolutionizing-plant-automation-through-real-time-data-insights]
- 53. BioWes-from design of experiment, through protocol to ... [https://biomedical-engineering-online.biomedcentral.com/articles/10.1186/s12938-016-0188-8]
- 54. A generalized platform for artificial intelligence-powered ... [https://www.nature.com/articles/s41467-025-61209-y]
- 55. 合成生物学与生物铸造厂:开启生物经济新时代 [https://www.mc-bio.cn/m/news_info.php?id=78]
- 56. Automated strain construction for biosynthetic pathway ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12611344/]
- 57. Triple Space-Time Yield in Discontinuous Antibody ... [https://www.mdpi.com/2306-5354/10/12/1391]
- 58. BioTech 2025: Key Trends, Innovations, and Challenges ... [https://biotechbreakthroughawards.com/biotech-2025-key-trends-innovations-and-challenges-shaping-the-future/]
- 59. Industrial Biotechnology Market 2025 Highlights [https://www.statifacts.com/outlook/industrial-biotechnology-market]
- 60. Biopharma Trends 2025 [https://www.bcg.com/publications/2025/biopharma-trends]
- 61. Five Key Trends Shaping Biopharma and Biotech in 2025 [https://www.ppd.com/blog/2025-biopharma-biotech-trends/]
- 62. Review article Carbon cycle of polyhydroxyalkanoates (CCP) [https://www.sciencedirect.com/science/article/abs/pii/S0013935125001550]
- 63. Academia Sinica Builds First Dual Carbon Fixation System ... [https://www.sinica.edu.tw/en/news_content/55/3304]
- 64. Mapping the Clinical Development Trajectory of Cell and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12447590/]
- 65. A generalized phase 1-2-3 design integrating dose ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10873567/]
- 66. Computational design of synthetic receptors with ... [https://www.nature.com/articles/s41551-025-01532-3]
- 67. PKBOIN-12: A Bayesian optimal interval Phase I/II design ... [https://arxiv.org/html/2312.15396v1]
- 68. A Drug Development Guide for Cell and Gene Therapies [https://www.ppd.com/blog/cell-and-gene-therapies-drug-development-guide/]
- 69. Engineering modular enzyme assembly: synthetic interface ... [https://pubs.rsc.org/en/content/articlehtml/2025/np/d5np00027k]
- 70. Galaxy-BioProd - Site web pour le PEPR B-BEST [https://www.pepr-bioproductions.fr/eng/funded-projects/cross-cutting-methodologies-and-tools/galaxy-bioprod]
- 71. DBTL bioengineering cycle for part characterization and ... [https://www.sciencedirect.com/science/article/pii/S2405896324017488]